Thieno-pyrimidine derivatives and uses thereof
一种药物、化合物的技术,应用在表皮生长因子受体的组合物领域,能够解决活性突变患者用途限制、剂量限制性毒性等问题,达到降低副作用的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The preparation of pharmaceutical compositions comprising active ingredients is well known in the art, for example by mixing, granulating or tabletting procedures. The active therapeutic ingredient is usually mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. For oral administration, the active agent is mixed with additives customary for this purpose, such as carriers, stabilizers or inert diluents, and converted by usual methods into a suitable administration form, such as tablets, coated tablets, hard or Soft gel capsules, water, alcohol or oil solutions, etc., as described above.
[0068] The amount of compound administered to a patient is less than that which would cause toxicity to the patient. In certain embodiments, the amount of compound administered to a patient is less than the amount that would cause the patient's blood levels to equal or exceed the toxic level of the compound. In the practice of this inve...
Embodiment 1
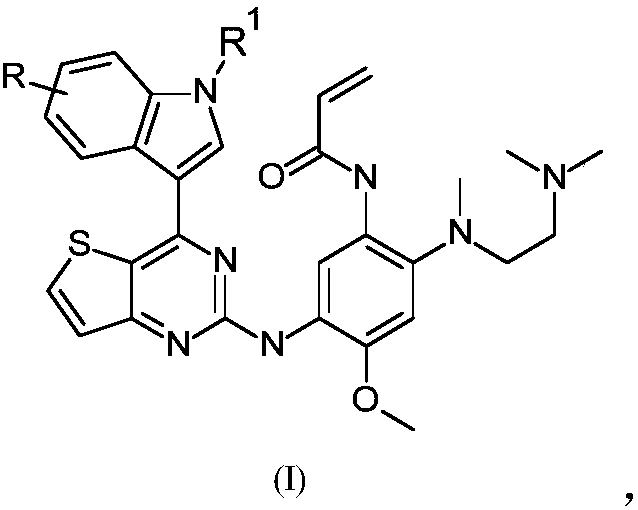
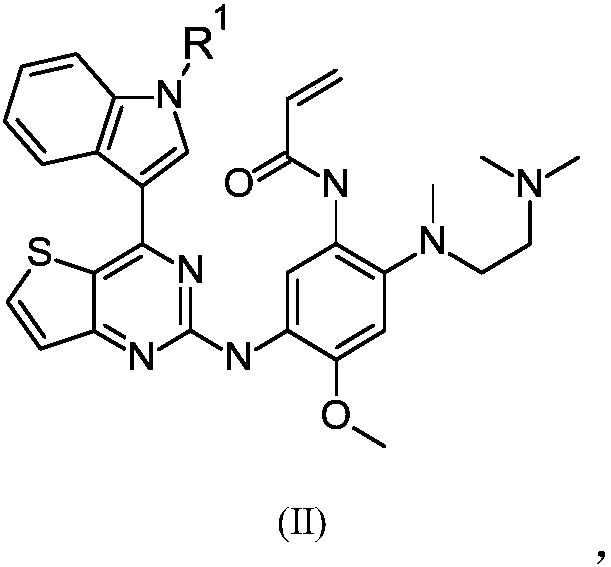
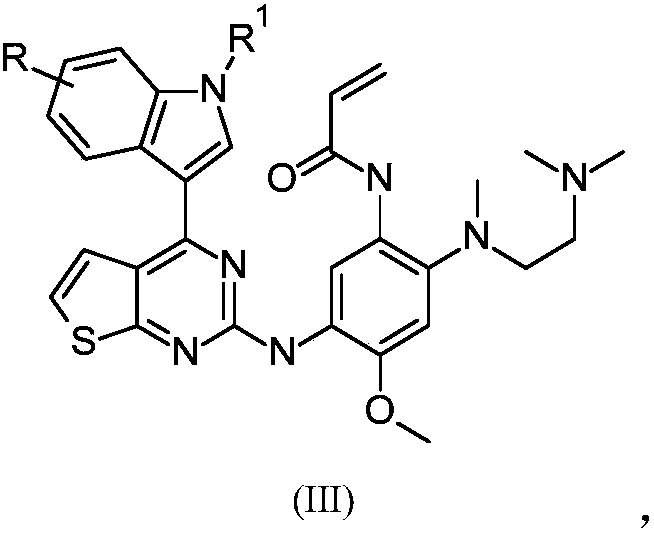
[0140] Example 1: N-[2-[2-(dimethylamino)ethyl-methyl-amino]-5-[-[[4-(1H-indol-3-yl)thiophene Synthesis of [3,2-d]pyrimidin-2-yl]amino]-4-methoxy-phenyl]prop-2-enamide (compound 1):
[0141]
[0142] Synthesis of Thieno[3,2-d]pyrimidine-2,4(1H,3H)-dione (Compound 102)
[0143] A mixture of methyl 3-amino-2-thiophenecarboxylate (13.48 g, 85.85 mmol) and urea (29.75 g, 0.43 mol) was heated at 190°C for 2 hours. The hot reaction mixture was then poured into sodium hydroxide solution and insoluble material was removed by filtration. The mixture was then acidified with 2N HCl solution, collected by filtration and air dried to afford the compound (9.62 g, 67%) as a white precipitate.
[0144] Synthesis of 2,4-Dichlorothieno[3,2-d]pyrimidine (Compound 103)
[0145] Compound 102 (8.5 g) was suspended with phosphorus oxychloride (130 mL). The mixture was heated at 100°C for 10 hours. POCl was removed under reduced pressure 3 . The mixture was dissolved in dichlorometha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com