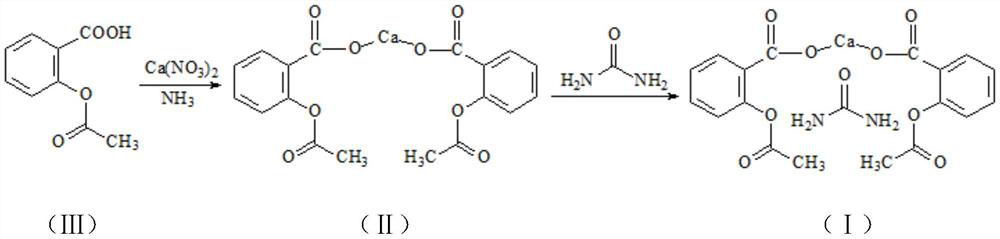
Preparation method of bis(2-acetoxybenzoic acid) calcium urea compound
A technology of acetoxybenzoic acid and compounds, which is applied in the field of preparation of dicalcium urea compounds, can solve problems such as poor quality of final products, unclarified reaction liquid, hidden dangers of safety, etc., and achieve stable product quality, easy control of reaction conditions, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 360kg of anhydrous (recovery) ethanol in a 1000L reactor, add 15.6kg of urea and 36.1kg of calcium nitrate under stirring, heat up to 40°C, stir and dissolve for 15 minutes, then add 72kg of acetylsalicylic acid compound (Ⅲ) and continue stirring Dissolve for 15 minutes, press filter, wash with 40kg of anhydrous (recovered) ethanol, cool the filtrate to 0±3°C while stirring, control the rate of ammonia flow, and pass about 6.8-6.9kg of ammonia within 60 minutes (based on the pH of the reaction solution ≤7.4), the reaction produces calcium acetylsalicylate compound (II) under this condition and continued to stir for 10 minutes, and the re-measured pH value was between 7 and 7.4. Then the temperature is raised gradually, and the reaction temperature is raised from 0±3°C to 15°C, and the heating time is 20 minutes. At this time, the pH of the reaction solution is ≤7; <7, keep warm for complexation reaction for 1 hour; then raise the temperature to 37±2°C, keep warm for...
Embodiment 2
[0028] Add 504kg of anhydrous (recovery) ethanol in a 1000L reactor, add 16.8kg of urea and 35.3kg of calcium nitrate under stirring, heat up to 50°C, stir and dissolve for 10 minutes, then add 72kg of acetylsalicylic acid compound (Ⅲ) and continue stirring Dissolve for 10 minutes, press filter, wash with 20kg of anhydrous (recovered) ethanol, cool the filtrate to 0±3°C under stirring, control the speed of ammonia flow, and pass in about 6.8-6.9kg of ammonia in 30 minutes (based on the pH of the reaction solution≤ 7.4 as the standard), the reaction generates calcium acetylsalicylate compound (II) under this condition and continued to stir for 10 minutes, and the re-measured pH value was between 7 and 7.4. Then the temperature is raised gradually, and the reaction temperature is raised from 0±3°C to 10°C for 30 minutes. At this time, the pH of the reaction solution is ≤7; <7, keep warm for complexation reaction for 1 hour; then raise the temperature to 37±2°C, keep warm for 1 h...
Embodiment 3
[0031] Add 432kg of anhydrous (recovery) ethanol in a 1000L reactor, add 14.4kg of urea and 34.4kg of calcium nitrate under stirring, heat up to 45°C, stir and dissolve for 13 minutes, then add 72kg of acetylsalicylic acid compound (Ⅲ) and continue stirring Dissolve for 13 minutes, press filter, wash with 30kg of anhydrous (recovered) ethanol, cool the filtrate to 0±3°C while stirring, control the rate of ammonia flow, and pass about 6.8-6.9kg of ammonia in 45 minutes (based on the pH of the reaction solution≤ 7.4 as the standard), the reaction generates calcium acetylsalicylate compound (II) under this condition and continued to stir for 10 minutes, and the re-measured pH value was between 7 and 7.4. Then the temperature is raised gradually, and the reaction temperature is raised from 0±3°C to 13°C for 25 minutes. At this time, the pH of the reaction solution is ≤7; then the temperature is raised from 13°C to 30±3°C for 25 minutes. <7, keep warm for complexation reaction for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com