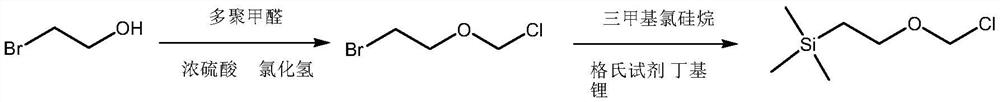
A kind of synthetic method of 2-(trimethylsilyl) ethoxymethyl chloride
The technology of an ethoxymethyl chloride and a synthetic method is applied in the synthesis field of 2-ethoxymethyl chloride, and can solve the problems of severe reaction, instability, low purity, etc., and achieves improved yield, enhanced stability, and improved reaction The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The first step of chloromethylation: Take 5L of n-hexane, 5kg of bromoethanol, 1300g of paraformaldehyde, and 800g of concentrated sulfuric acid, put them into a 20L reaction bottle, and start stirring. The system was cooled to -5-0°C, and HCL gas was introduced. Introduce slowly at the beginning, and after about 10 minutes, speed up the aeration rate and control the internal temperature to 5-8°C. When the reaction solution becomes clear, slow down the aeration rate again. Gas phase monitoring, until the raw material is less than 5%, stop ventilation (about 2450g of shared hydrogen chloride). Add 10 g of stabilizer to the reaction liquid, and stir for 5 min. The layers were separated, and the lower layer was extracted once with 2 L of n-hexane, and the organic phases were combined. Concentrate under reduced pressure at 50°C (recovery of n-hexane is applied mechanically), until there are no bubbles and no reflux to obtain a crude product. 60-90°C, water pump rectifica...
Embodiment 2
[0048] The first step of chloromethylation: Take 5L of n-hexane, 5kg of bromoethanol, 1300g of paraformaldehyde, and 800g of concentrated sulfuric acid, put them into a 20L reaction bottle, and start stirring. The system was cooled to -5-0°C, and HCL gas was introduced. Introduce slowly at the beginning, and after about 10 minutes, speed up the aeration rate and control the internal temperature to 5-8°C. When the reaction solution becomes clear, slow down the aeration rate again. Gas phase monitoring, until the raw material is less than 5%, stop ventilation (about 2490g of shared hydrogen chloride). Add 10 g of stabilizer to the reaction liquid, and stir for 5 min. The layers were separated, and the lower layer was extracted once with 2 L of n-hexane, and the organic phases were combined. Concentrate under reduced pressure at 50°C (recovery of n-hexane is applied mechanically), until there are no bubbles and no reflux to obtain a crude product. 60-90°C, water pump rectifica...
Embodiment 3
[0052] The first step of chloromethylation: Take 10L of n-hexane, 10kg of bromoethanol, 2600g of paraformaldehyde, and 1600g of concentrated sulfuric acid, put them into a 50L reaction kettle, and start stirring. The system was cooled to -5-0°C, and HCL gas was introduced. Introduce slowly at the beginning, and after about 10 minutes, speed up the aeration rate and control the internal temperature to 5-8°C. When the reaction solution becomes clear, slow down the aeration rate again. Gas phase monitoring, until the raw material is less than 5%, stop ventilation (about 5080g of shared hydrogen chloride). Add 25 g of stabilizer to the reaction liquid, and stir for 5 min. The layers were separated, and the lower layer was extracted once with 4 L of n-hexane, and the organic phases were combined. Concentrate under reduced pressure at 50°C (recovery of n-hexane is applied mechanically), until there are no bubbles and no reflux to obtain a crude product. 60-90°C, water pump rectif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com