Recombinant oncolytic adenovirus, recombinant oncolytic adenovirus vector for preparing same, construction method of recombinant oncolytic adenovirus vector and applications of recombinant oncolytic adenovirus and recombinant oncolytic adenovirus vector
A technology of oncolytic adenovirus and construction method, which is applied in the field of recombinant oncolytic adenovirus vector and its construction, can solve the problems of tumor recurrence and metastasis, failure to fundamentally cure tumor, patient death, etc., and achieve reduction of recurrence and metastasis, good The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
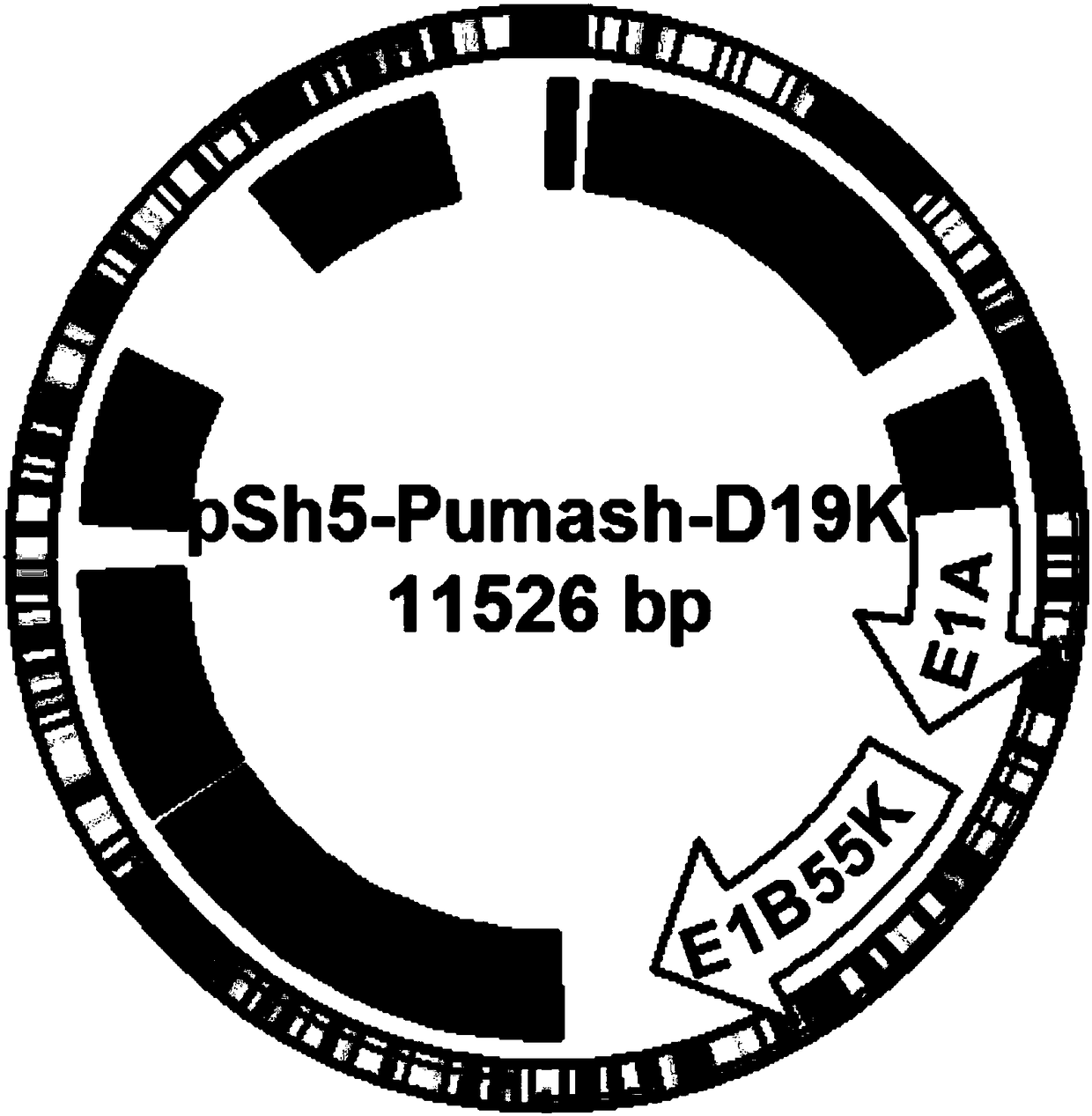
[0051] Example 1 Construction, Amplification, Purification and Measurement of Particle Titer and Infection Titer of Recombinant Oncolytic Adenoviral Vector
[0052] 1. Modification of adenovirus backbone
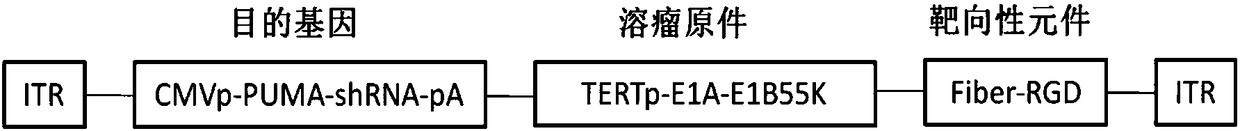
[0053] In order to increase the infection efficiency of tumor cells with low Saatchi virus-adenovirus receptor (CAR) expression, the RGD-4C short peptide sequence was introduced into the fiber (Fiber) gene of the adenovirus plasmid, and the selected positions were 456T and 457P of HIloop In between, the targeting backbone plasmid pAdbone5RGD was constructed.
[0054] 2. Construction of oncolytic adenoviral vectors carrying therapeutic gene shRNA and PUMA and control adenoviral vectors
[0055] Construction strategy: clone the target gene (shRNA anti-tumor stem cell marker ALDH1A1 gene and pro-apoptosis gene PUMA gene) into the multiple cloning site of the shuttle plasmid pShuttle-CMV (the shuttle plasmid contains CMVp and pA signal sequences) to obtain Plasmid pSh5-Pumash;...
Embodiment 2
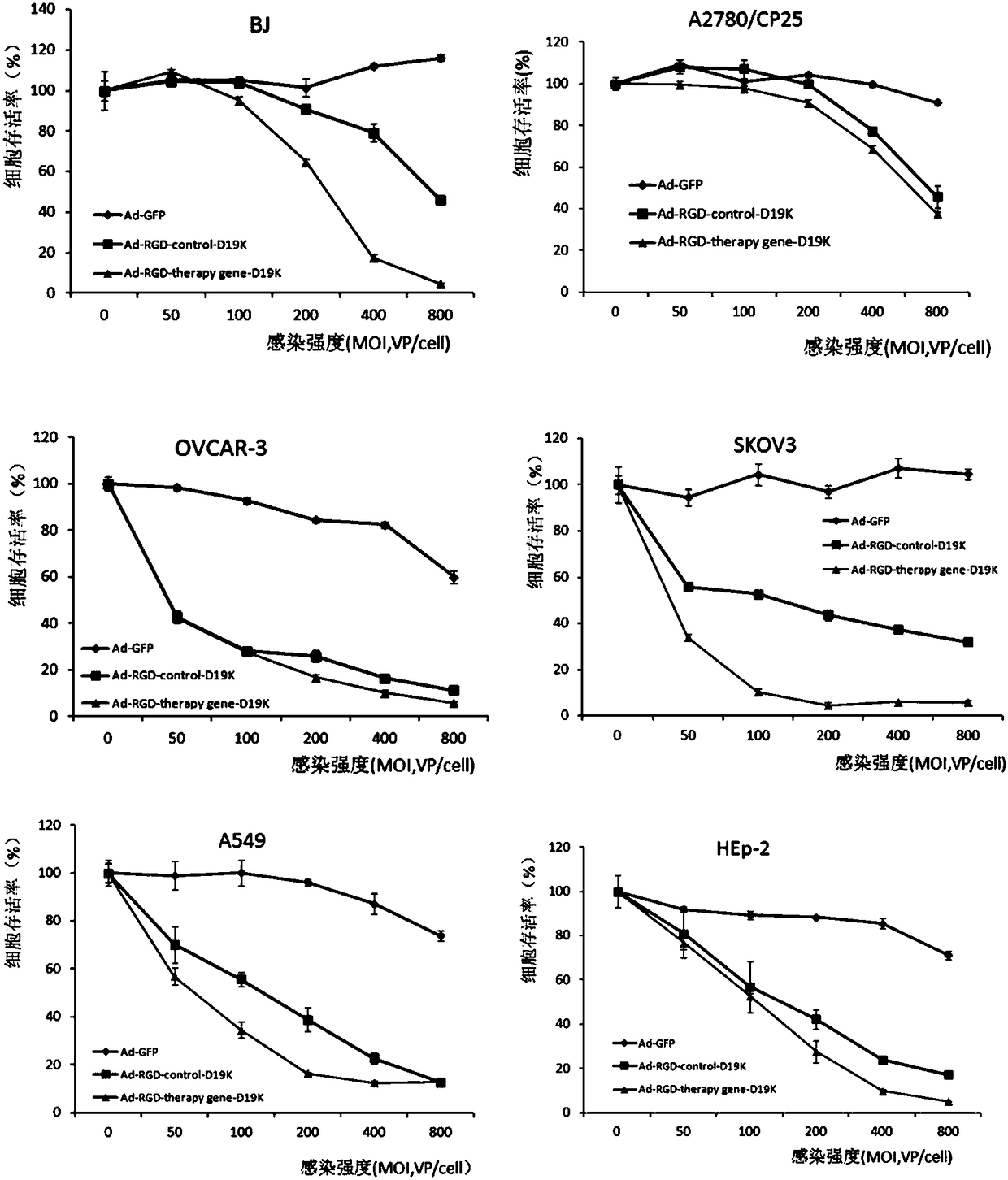
[0056] Example 2 Recombinant oncolytic adenovirus related experiments
[0057] 1. Amplification and purification of recombinant (oncolytic) adenovirus and determination of particle titer and infection titer
[0058] The prepared recombinant (oncolytic) adenovirus was massively amplified in human embryonic kidney 293 cells, purified by a special cesium chloride density gradient centrifugation method, and the number of adenovirus particles was measured by a digestion method, and the adenovirus concentration was measured by a limiting dilution method. Infectious titer, according to the ratio of particle titer / infectious titer to judge the proportion of live virus in pure adenovirus, the ratio of the above four recombinant oncolytic adenoviruses is <100, which is in line with the requirements of the US FDA and Chinese biological products on adenovirus requirements (as shown in Table 1).
[0059] Table 1 Particle titer, infection titer and ratio of recombinant (oncolytic) adenovir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com