Glycoconjugate containing stn or f-stn and its preparation method and application in antitumor vaccine
A technology of glycoconjugates and vaccines, applied in the application field of anti-tumor vaccines, can solve the problems of poor reproducibility of KLH protein and limited clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
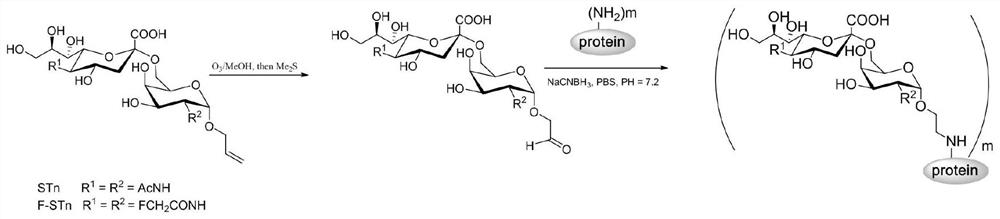
[0051] The invention provides a preparation method of the glycoconjugate, comprising the following steps:
[0052] (1) A compound as shown in formula III is oxidized by ozone to obtain a disaccharide containing an aldehyde group;
[0053]
[0054] (2) Coupling the aldehyde group-containing disaccharide obtained in step (1) with a carrier under the condition of reductive amination to obtain a glycoconjugate containing STn or F-STn.
[0055] In the present invention, the concentration of the ozone is preferably 30-50 mg / L, more preferably 40 mg / L. The temperature of the ozone oxidation is preferably -72°C. The time for the ozone oxidation is preferably 10-30 min, more preferably 15-25 min, most preferably 20 min. The ozonation oxidizes the alkene group in formula III to an aldehyde group.
[0056] In the present invention, the conditions of the reductive amination preferably add NaBH 3 CN; The disaccharide containing aldehyde group, carrier and NaBH 3 The mass ratio of C...
Embodiment 1
[0067] Preparation method for coupling hapten F-STn or STn with carrier protein to form conjugate
[0068] Dissolve the hapten (F-STn or STn, 10mg) in 2mL of anhydrous methanol, and pass through the air containing ozone at -72°C (ozone concentration is 50mg / L), when the system turns blue (about 10~ After 30 minutes), the ozone flow was stopped, and the system was still blue after 10 minutes. Nitrogen was introduced into the reaction system for about 10 minutes to remove excess ozone. 0.5 mL of dimethyl sulfide was added dropwise, and then the temperature of the reaction system was naturally raised to room temperature. After 2 hours, the solvent was removed from the reaction system under vacuum to obtain a hapten containing an aldehyde group. The latter was dissolved together with 5 mg of protein CRM197 in 10 mM phosphate buffer at pH 7.2, 7.5 mg of sodium cyanoborohydride was added, and reacted on a shaker at room temperature for 24 h. The desired glycoprotein conjugate STn-...
Embodiment 2
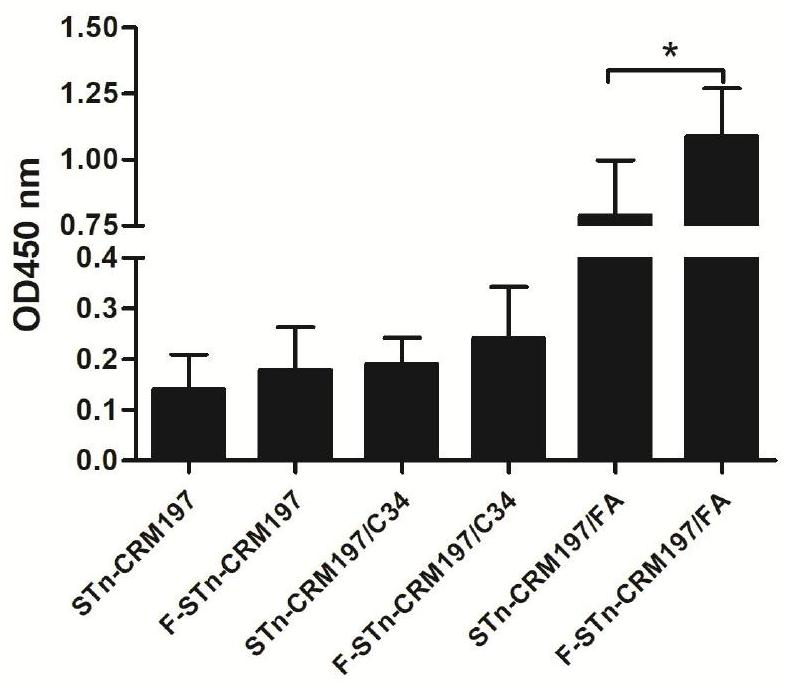
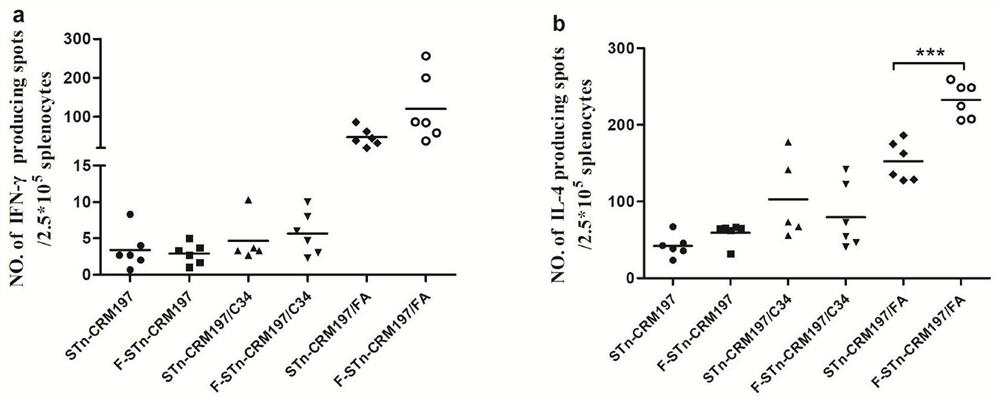
[0070] Glycoprotein conjugate STn-CRM197 or F-STn-CRM197 immunological activity test
[0071] 1. Test materials and sources
[0072] 1. Test compound: the glycoprotein conjugate prepared in Example 1 of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com