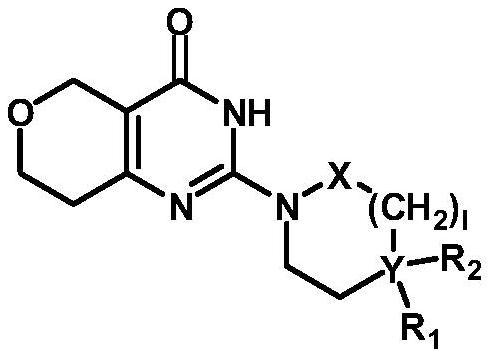
Novel dihydropyranopyrimidinone derivatives and uses thereof
A pyrimidine and pyran technology, applied in the field of novel dihydropyranopyrimidinone derivatives, can solve problems such as increased concentration of axin, decreased Wnt signal transduction, and concentration of destruction complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
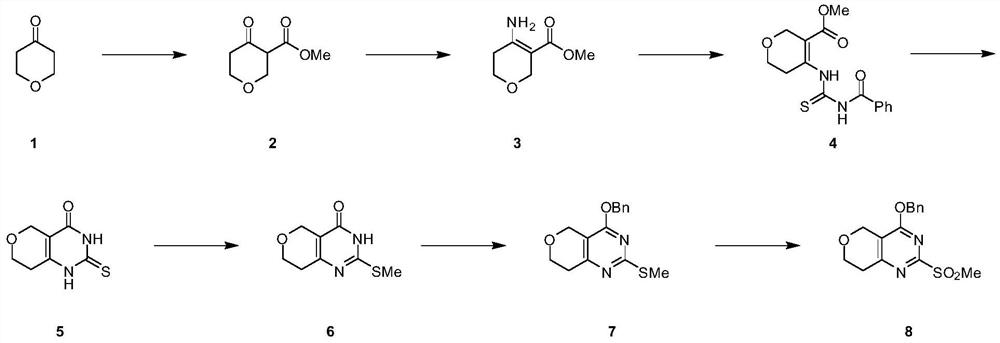
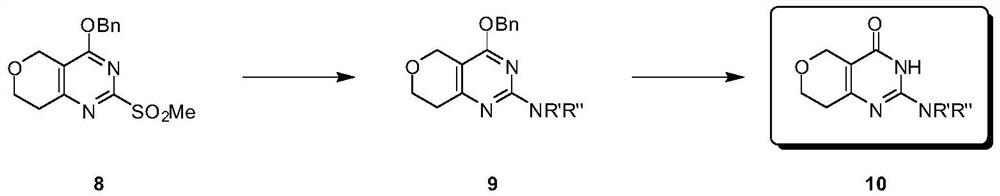
[0119] Preparation 1: 4-(benzyloxy)-2-methylsulfonyl)-7,8-dihydro-5H-pyrano[4,3- d] pyrimidine (I-7)
[0120] 4-(Benzyloxy)-2-methylsulfonyl)-7,8-dihydro-5H-pyrano[4,3-d]pyrimidine (I-7) was prepared as an intermediate based on the following reaction scheme For the synthesis of dihydropyranopyrimidinone derivatives.
[0121]
[0122] 1.1. 4-Oxotetrahydro-2H-pyran-3-carboxylic acid methyl ester (I-1)
[0123] To a mixture of NaH (1.97 g, 44.96 mmol) in THF (100 ml) was added dihydro-2H-pyran-4(3H)-one (3 g, 29.96 mmol) dropwise in an ice bath. After stirring for 20 min, dimethyl carbonate (3.8 ml, 44.96 mmol) was added. The reaction mixture was stirred at room temperature for 3 h. The reaction mixture was poured into a mixture of diethyl ether / 1N HCl with stirring. The organic layer was separated and washed with Na 2 SO 4 It was dried, concentrated under reduced pressure, and the residue was purified by column chromatography to give the desired product I-1 (1.5 g...
preparation example 2
[0143] Preparation 2: 1-(2,6-difluoro-4-(2-methoxyethoxy)phenyl)piperazine hydrochloride (I-8a) and 1-(2,6- Difluoro-4-(2-(piperidin-1-yl)ethoxy)phenyl)piperazine dihydrochloride (I-8b)
[0144]
[0145] 2.1. tert-butyl 4-(2,6-difluoro-4-(2-methoxyethoxy)phenyl)piperazine-1-carboxylate
[0146] tert-butyl 4-(2,6-difluoro-4-hydroxyphenyl)piperazine-1-carboxylate (2 g, 6.363 mmol), 1-bromo-2-methoxyethane (0.72 ml, 7.635mmol) and K 2 CO 3 (2.64g, 19.09mmol) was heated to 60°C to 65°C. After stirring for 16 h, the mixture was cooled to room temperature, diluted with EtOAc, and washed three times with water. The organic layer was washed with Na 2SO 4 Drying and concentration under reduced pressure gave the desired product (2.39 g) as a yellow solid.
[0147] LC-MS (ESI, m / z) = 373.2 (M+H + ).
[0148] 2.2-1. 1-(2,6-Difluoro-4-(2-methoxyethoxy)phenyl)piperazine hydrochloride
[0149] To a solution of the compound obtained in Preparation 2.1 (2.39 g, 6.363 mmol) ...
preparation example 3
[0154] Preparation 3: 3,5-difluoro-4-(piperazin-1-yl)phenol hydrochloride
[0155] By removing the protecting group of tert-butyl 4-(2,6-difluoro-4-hydroxyphenyl)piperazine-1-carboxylate, the desired product was prepared following a method similar to that described in Preparation 2.2-1.
[0156] LC-MS (ESI, m / z) = 215.1 (M+H + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com