Synthesis method of palbociclib intermediate
A synthesis method and Palbociclin technology, applied in the field of synthesizing Palbociclin intermediates, can solve the problems of complicated product obtaining process, inconvenient recovery, complicated product extraction and purification of hydrogenation reaction, etc., and achieve environmental protection improvement, product Yield and Purity Improvement, Product Yield and Purity Improvement Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
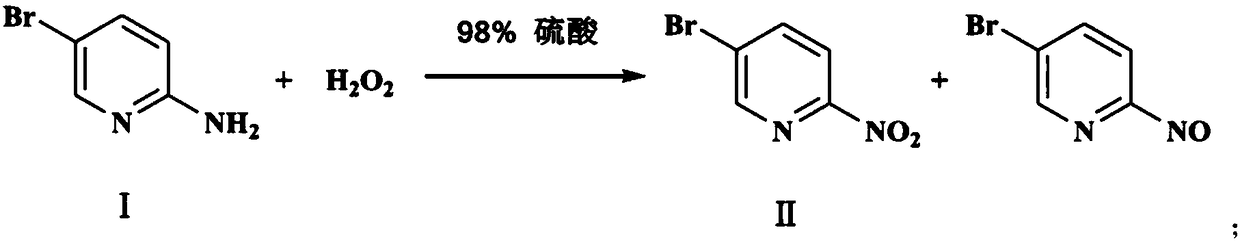
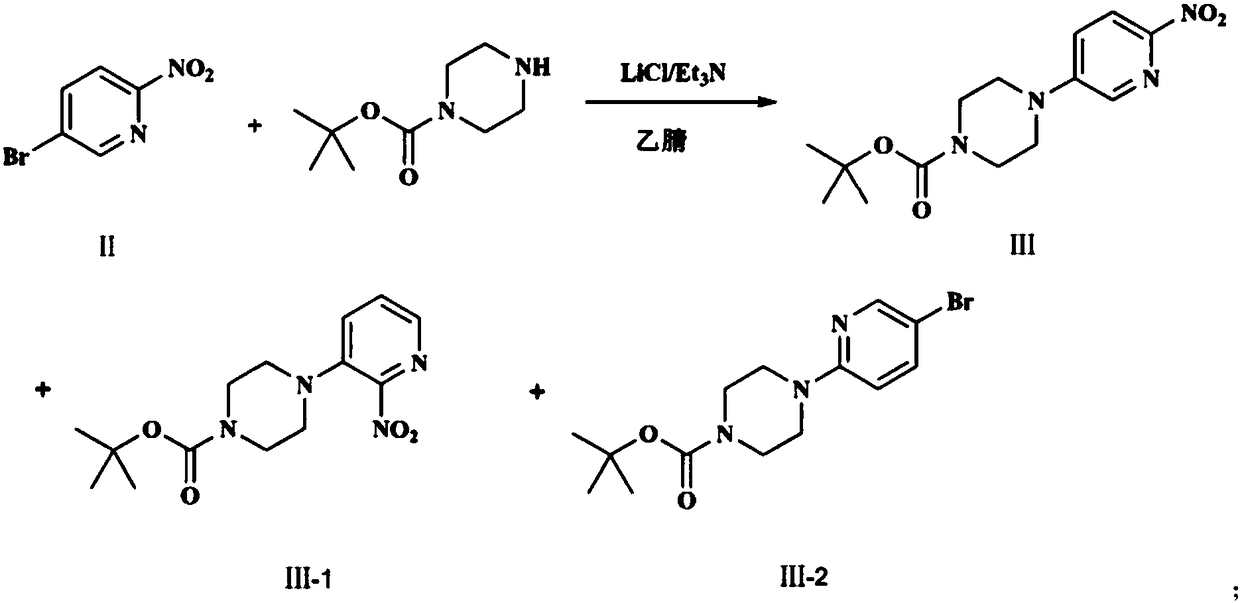
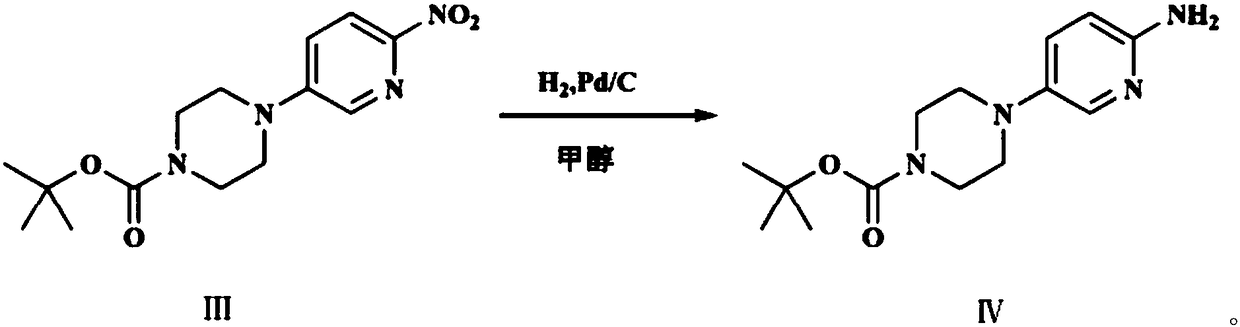
[0022] Synthesize the desired product through the following three steps, as follows:
[0023] 1, Synthesis of 2-nitro-5-bromopyridine (II)
[0024] Add 160ml of 30% hydrogen peroxide to a 1000ml three-necked reaction flask, use a low-temperature reaction bath to cool down to within 15°C, and use a constant pressure dropping funnel to drop 320ml of 98% concentrated sulfuric acid into the hydrogen peroxide in the reaction flask. The temperature of the solution in the bottle is controlled at 5 In the range of -15°C, solution B was obtained after the dropwise addition was completed. At the same time, add 160ml of 98% concentrated sulfuric acid to another 1000ml three-necked reaction flask, use a low-temperature reaction bath to cool down, add 60.00g of 2-amino-5-bromopyridine in batches, control the temperature within the range of 5-15°C, and obtain a solution after adding a.
[0025] Both solutions A and B have been prepared. Use a constant pressure dropping funnel to add solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com