Traditional Chinese medicine toothpaste containing radix ilicis asprellae and folium artemisiae argyi and preparation method
A technology of traditional Chinese medicine toothpaste and Gangmei, which is applied in the field of traditional Chinese medicine toothpaste containing Gangmei and Artemisia argyi, can solve the problem that toothpaste does not have the ability to control recurrent oral ulcers, gingival swelling and pain, and achieve medical cost saving, economic burden, and clinical benefits. The effect of reducing symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
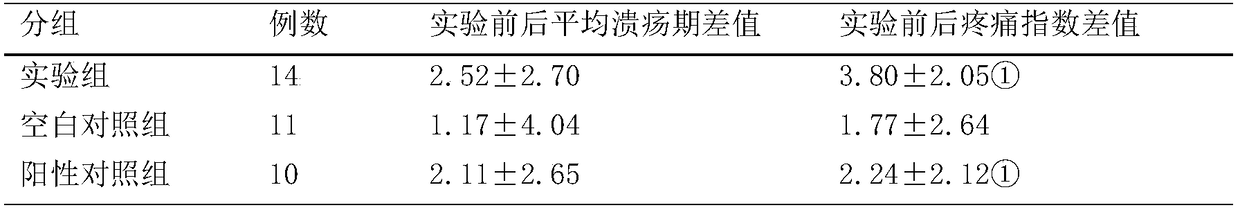
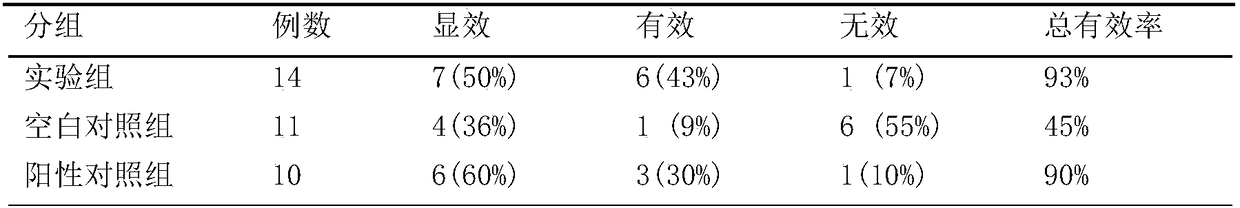
[0041] A kind of traditional Chinese medicine toothpaste containing Gangmei and Folium Artemisiae Argyi provided by the present embodiment, in parts by mass, includes: 50 parts of calcium hydrogen phosphate dihydrate, 30 parts of deionized water, 20 parts of sorbitol, 5 parts of glycerin, silicon dioxide 1 part, 1 part essence, 3 parts sodium lauryl sulfate, 0.5 part sodium saccharin, 1 part cellulose gum, 0.5 part tetrasodium pyrophosphate carrageenan, 0.1 part root extract powder, 0.5 part leaf extract powder.
[0042] The preparation method is as follows:
[0043] S1. First, put the root extract powder, Artemisia argyi extract powder, silicon dioxide, sodium lauryl sulfate, tetrasodium carrageenan pyrophosphate, and sodium saccharin in a pre-dissolved pot with deionized water and stir for 15 minutes to dissolve evenly. Then transfer to the paste pot;
[0044] S2. Add sorbitol, glycerin, calcium hydrogen phosphate dihydrate and cellulose gum into the paste making pot, then ...
Embodiment 2
[0048] A kind of traditional Chinese medicine toothpaste containing Gangmei and Folium Artemisiae Argyi provided by the present embodiment comprises, in parts by mass: 40 parts of calcium hydrogen phosphate dihydrate, 20 parts of deionized water, 10 parts of sorbitol, 10 parts of glycerin, silicon dioxide 5 parts, 3 parts essence, 1 part sodium lauryl sulfate, 1 part sodium saccharin, 0.5 part cellulose gum, 1 part tetrasodium pyrophosphate carrageenan, 0.5 part root extract powder, 0.1 part leaf extract powder.
[0049] The preparation method is as follows:
[0050] S1. First, put the root extract powder, mugwort leaf extract powder, silicon dioxide, sodium lauryl sulfate, tetrasodium carrageenan pyrophosphate, and sodium saccharin in a pre-dissolved pot with deionized water and stir for 45 minutes to dissolve evenly. Then transfer to the paste pot;
[0051] S2. Add sorbitol, glycerin, calcium hydrogen phosphate dihydrate and cellulose gum into the paste making pot, then add...
Embodiment 3
[0055] A kind of traditional Chinese medicine toothpaste containing Gangmei and Artemisia argyi provided by the present embodiment, in parts by mass, comprises: 45 parts of calcium hydrogen phosphate dihydrate, 25 parts of deionized water, 15 parts of sorbitol, 8 parts of glycerin, silicon dioxide 3 parts, 2 parts of essence, 2 parts of sodium lauryl sulfate, 0.7 parts of sodium saccharin, 0.8 parts of cellulose gum, 0.7 parts of tetrasodium pyrophosphate carrageenan, 0.25 parts of root extract powder, 0.25 parts of mugwort leaf extract powder.
[0056] The preparation method is as follows:
[0057] S1. Stir the root extract powder, leaf extract powder, silicon dioxide, sodium lauryl sulfate, tetrasodium carrageenan pyrophosphate, and sodium saccharin in a pre-dissolved pot with deionized water for 30 minutes to dissolve evenly. Then transfer to the paste pot;
[0058] S2. Add sorbitol, glycerin, calcium hydrogen phosphate dihydrate and cellulose gum into the paste making pot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com