Preparation technology of ropivacaine mesoporous bioactive glass composite microspheres
A technology of bioactive glass and composite microspheres, which can be used in drug combinations, organic active ingredients, microcapsules, etc., and can solve problems such as lack of research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
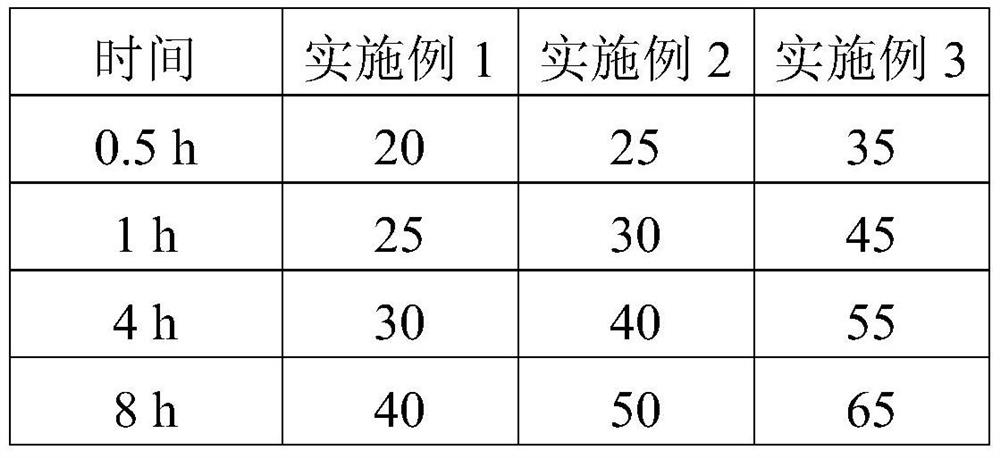
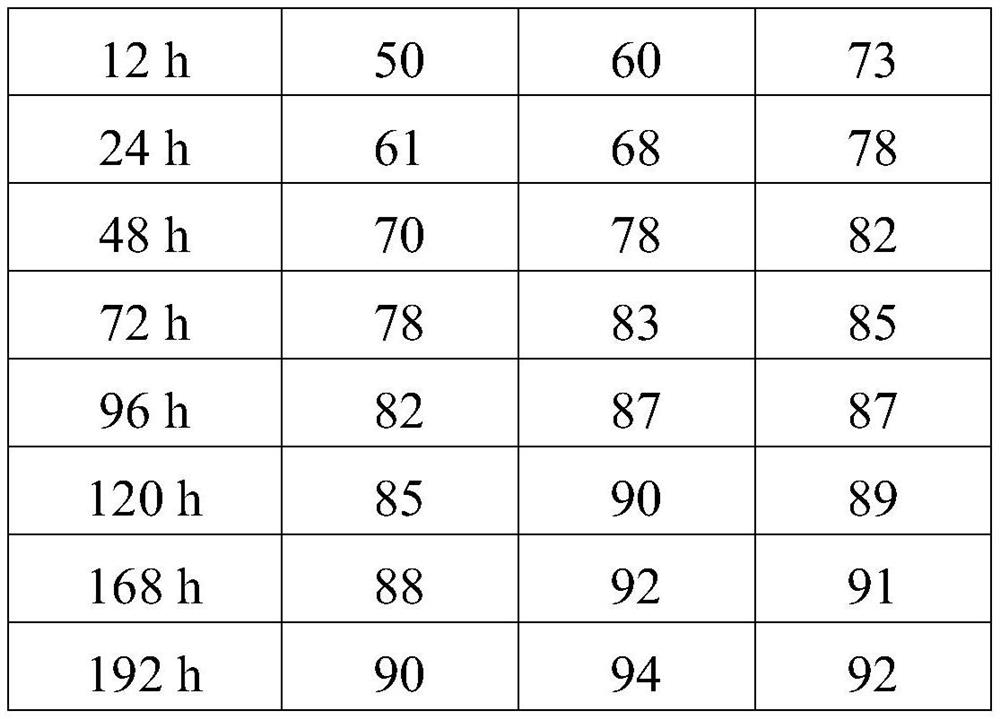
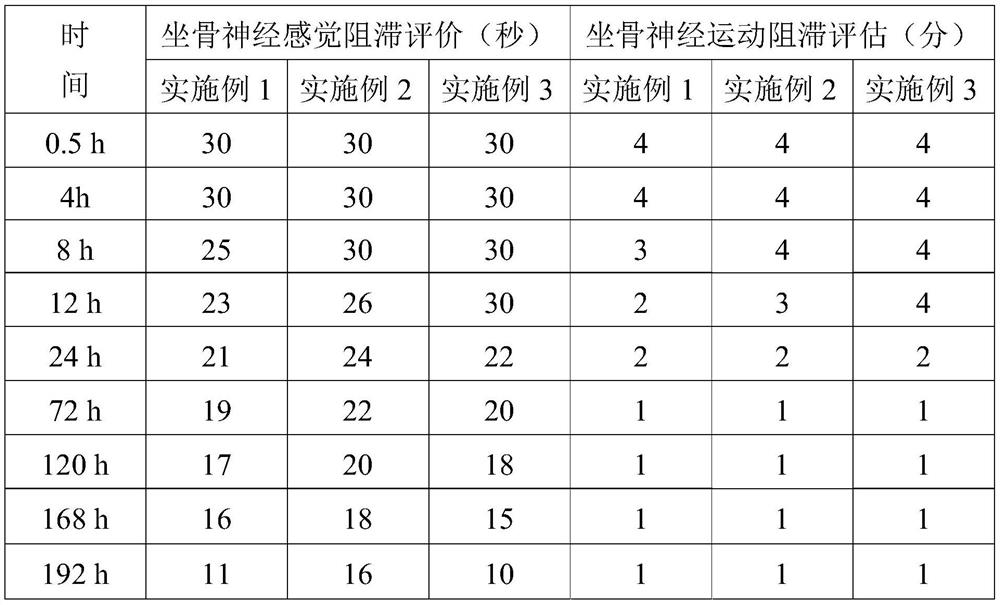
Embodiment 1
[0032] Example 1: The specific preparation method of the ropivacaine mesoporous bioactive glass composite microspheres provided in this example is as follows:
[0033] Step 1: SiO 2 Preparation of colloidal crystal templates
[0034] Monodisperse SiO 2 Preparation of Microspheres: Using A method for the preparation of monodisperse SiO by an alkaline hydrolysis process of ethyl orthosilicate (TEOS) 2 microsphere suspension. Weigh 251.2g absolute ethanol, 118.25g deionized water and 34g NH 3 ·H 2 O was placed in an Erlenmeyer flask, and after mixing evenly under magnetic stirring, 27.7g TEOS was added, and magnetic stirring was continued for 3h to obtain SiO 2 Suspension of particles.
[0035] SiO 2 Preparation of colloidal crystal template: firstly, the SiO prepared above 2 The suspension of particles was rotovaped to remove excess solvent, which was then placed in a flat-bottomed vessel and centrifuged to remove excess solvent, resulting in large, disordered SiO 2 c...
Embodiment 2
[0044] Example 2: The preparation method is the same as in Example 1, except that in step 5, the PEG with a molecular weight of 6,000 is replaced with PEG with a molecular weight of 10,000, and the pressurized plane is made of glass. The pressurized pressure was 0.054 MPa.
Embodiment 3
[0045] Example 3: The preparation method is the same as that of Example 1, except that in step 5, the PEG with a molecular weight of 6,000 is replaced with PEG with a molecular weight of 20,000, and ceramics are used for the pressing plane. The pressurized pressure is 0.08 MPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Mohs hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com