Method for enhancing anticancer capability of cells and enhanced type cells obtained by the method
An enhanced, cellular technology, applied in the biological field, to achieve the effect of enhancing the killing effect and enhancing the ability to resist liver cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
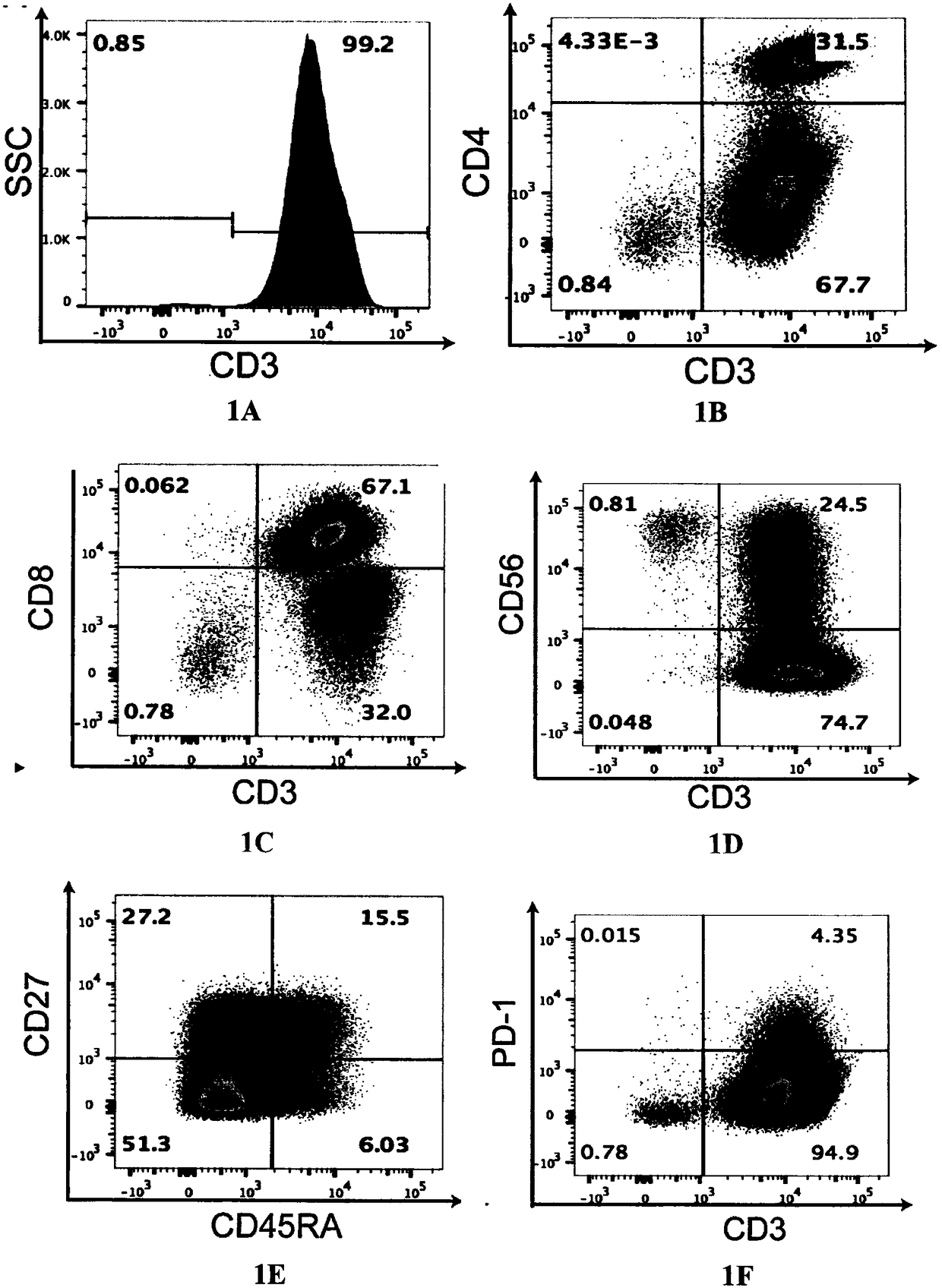
[0052] Example 1 CIK cell culture
[0053] Human peripheral blood was obtained from patients with hepatocellular carcinoma in Beijing Shijitan Hospital, and the written consent of the patients with liver cancer was obtained and approved by the Hospital Ethics Committee.
[0054] The culture method of CIK cells was based on the protocol of previous studies (Schmidt-wolf IG et al., Use of a SCIDmouse / human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991; 174 (1 ): 139-149). In short, 40ml of peripheral blood was obtained from a patient with hepatocellular carcinoma on day 0, and PBMC cells were separated by Ficoll-Hypaque gradient centrifugation, and the PBMC cells were suspended in GT-T551 serum-free medium (TAKARA company, Japan), and added 5% human AB plasma, 1000 U / mL IFN-γ (PeproTech). On day 1, 50 ng / mL anti-CD3 antibody (eBiosciences) and 100 U / mL recombinant human IL-2 (eBiosciences) were added to the c...
Embodiment 2
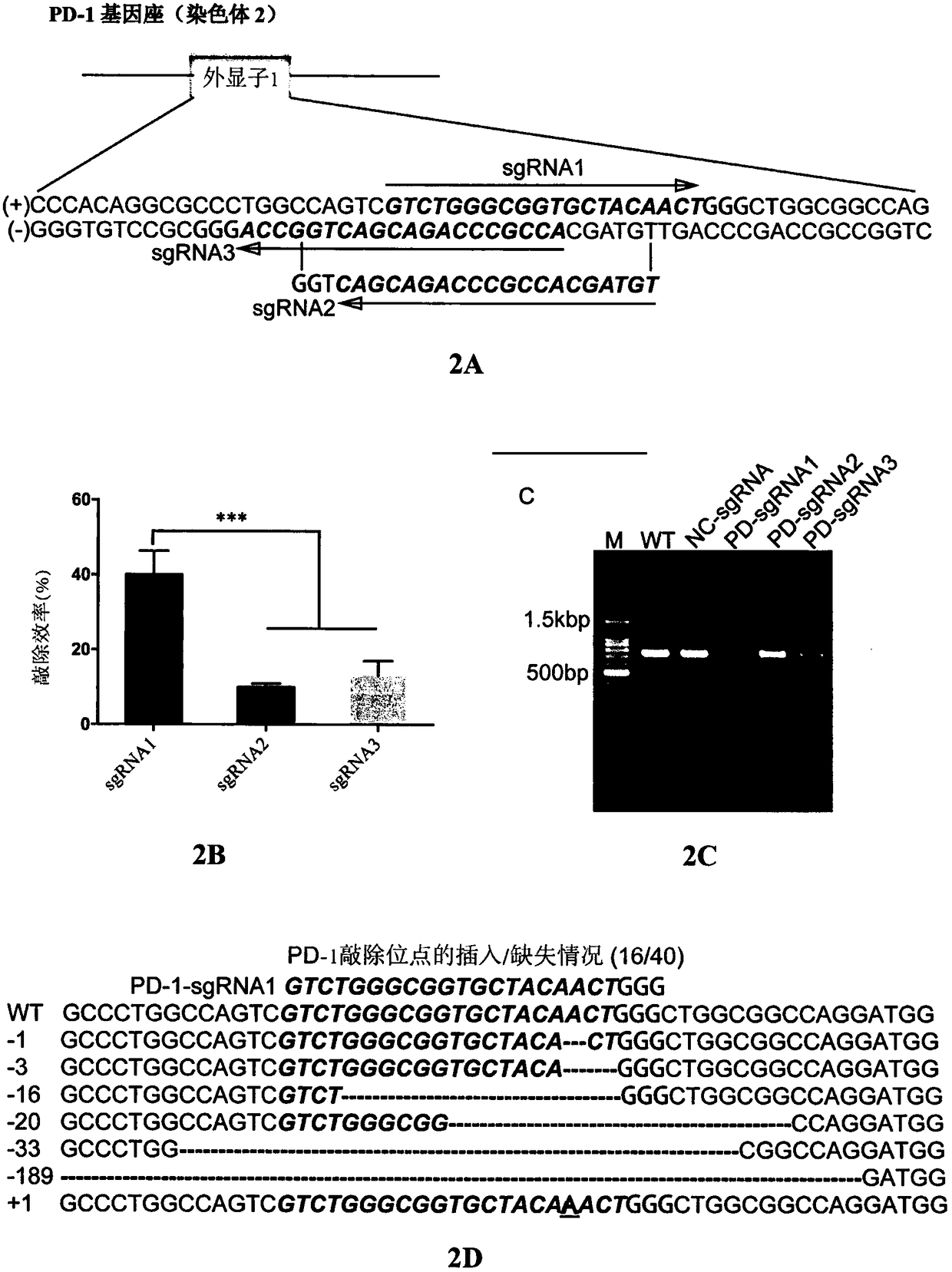
[0056] Example 2 sgRNA design and in vitro T7 transcription of sgRNA
[0057] The PD-1 exon 1 sequence was obtained from NCBI, and two CRISPR design tools (http: / / crispr.mit.edu and https: / / portals.broadinstitute.org / gpp / public / ) were used to design sgRNA, Three gRNAs were comprehensively selected:
[0058] SEQ ID NO.1 (PD-1 sgRNA1): GTCTGGGCGGTGCTACAACT
[0059] SEQ ID NO.2 (PD-1 sgRNA2): TGTAGCACCGCCCAGACGAC
[0060] SEQ ID NO.3 (PD-1 sgRNA3): ACCGCCCAGACGACTGGCCA
[0061] Use pX330 plasmid (Addgene plasmid #4223) as a template, T7 promoter + 20bp targeting sequence oligonucleotide + 20bp sgRNA scaffer as forward primer (TAATACGACTCACTATAGNNNNNNNNNNNNNNNNNNNNNN (20bp target sequence) GTTTTAGAGCTAGAAATAGC). Specifically, the three forward primers are:
[0062] SEQ ID NO.4 (forward primer of PD-1 sgRNA1):
[0063]
[0064] SEQ ID NO.5 (forward primer of PD-1 sgRNA2):
[0065]
[0066] SEQ ID NO.6 (forward primer of PD-1 sgRNA3):
[0067]
[0068] The reverse p...
Embodiment 3
[0071] Example 3 Preparation of PD-1 knockout CIK cells and detection of knockout
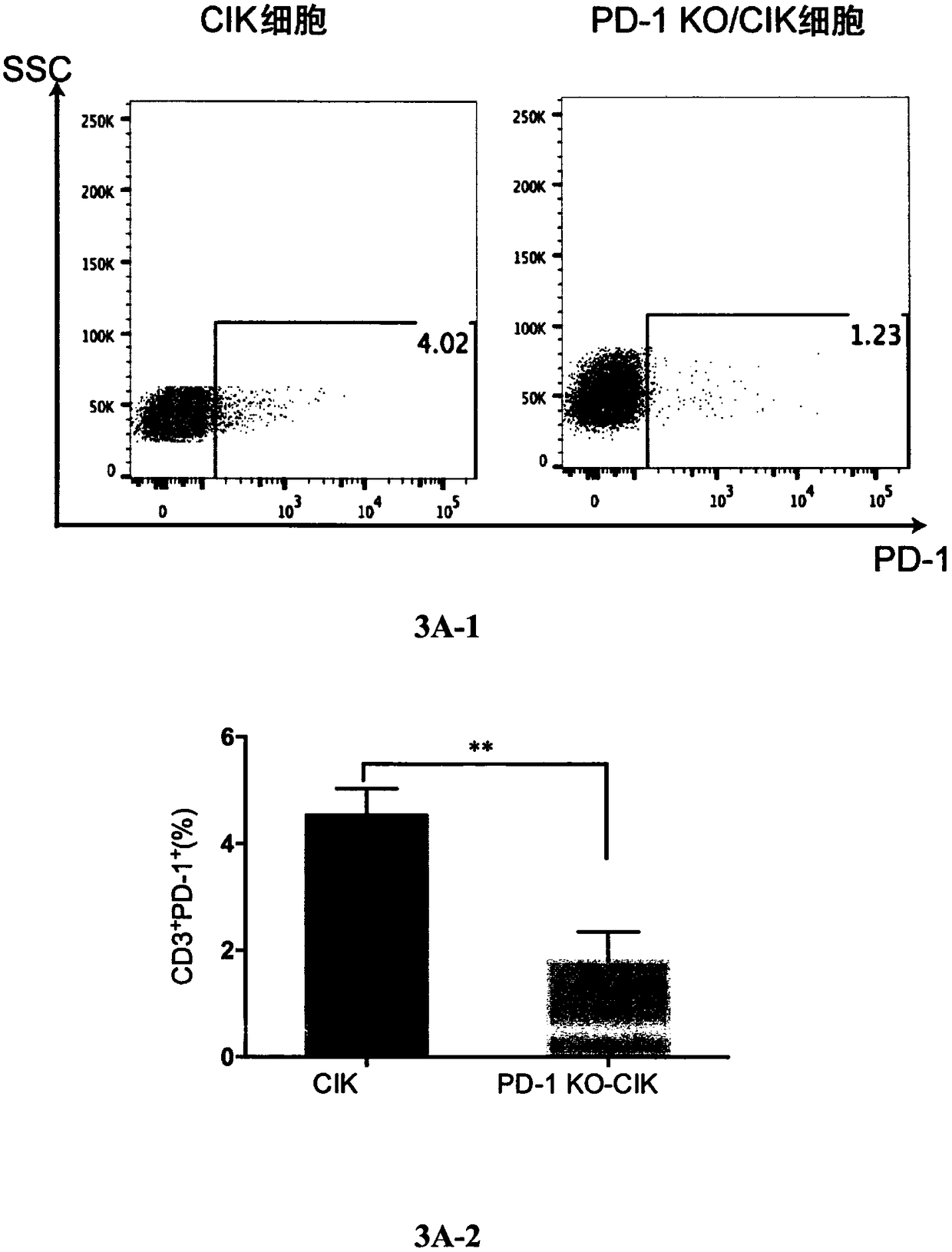
[0072] Using P3 Primary Cell 4D-Nucleofector X Kit (Lonza, Germany) through 4D-Nucleofector System X (Lonza, Germany), three kinds of targeted PD- 1 Exon 1 of any sgRNA, electroporated 5×10 6 CIK cells prepared in Example 1. Preheat 50ml of serum-free GT-T551 medium before electroporation, collect CIK cells, centrifuge and add 70μl electrotransfer solution to resuspend the cells, meanwhile add Cas9 protein and sgRNA to 30μl electrotransfer solution, incubate at room temperature for 10 minutes, mix Cas9 protein and The sgRNA mixture was added to the cell suspension, and electroporation was performed with the EO-115 electroporation program using a Lonza 4D-Nucleofector X Unit electroporation instrument. After electroporation, let the cells rest for 24 hours, then resuspend the cells in 2 ml of pre-warmed GT-T551 medium containing 5% human AB plasma and 100 U / mL recombinant human IL-2, and tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com