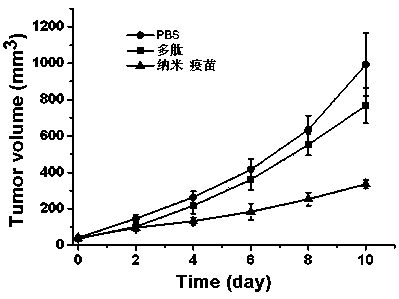
DC targeted nano vaccine applied to immunotherapy of liver cancer and preparation method thereof
A nano-vaccine and immunotherapy technology, which is applied in the field of nano-vaccine immunotherapy for liver cancer and its preparation, can solve the problems of poor solubility, achieve the effect of increasing endocytosis, increasing the effect of immunotherapy, and making the preparation method easy to repeat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1, the preparation of mannose modified ethylene glycol chitosan:
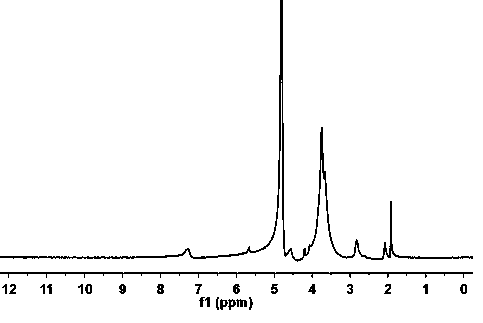
[0029] Dissolve ethylene glycol chitosan (205 mg, molecular weight 50,000) and 4-isosulfuric acid phenyl-alpha-D-mannoside (91 mg) in water. At this time, the structural units of ethylene glycol chitosan and 4- The molar ratio of isosulfuric acid phenyl-alpha-D-mannoside is 1:0.3, after adding water, the concentration of ethylene glycol chitosan is 2mg / ml, stirred and reacted at room temperature for 24h, dialyzed against water, freeze-dried, Obtain mannose-modified ethylene glycol chitosan, and the product obtained is denoted as MAN-GCTS, and its NMR characterization is shown in figure 1 , Depend on figure 1 It can be seen that MAN has been successfully introduced into GCTS.
[0030] Step 2, the preparation of nano vaccine:
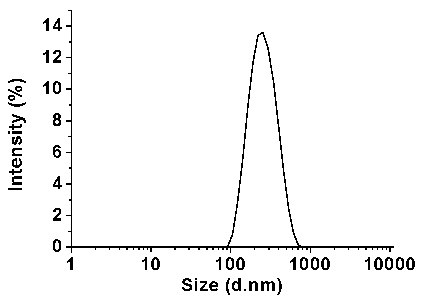
[0031] GPC-3 144-152 (1 mg) was dissolved in 1 mL phosphate-buffered saline (PBS, pH = 7.4); the MAN-GCTS (10 mg) prepared in Step 1 was dissolved in 5 ml water, and then a...
Embodiment 2
[0038] Step 1, the preparation of mannose modified ethylene glycol chitosan:
[0039] Dissolve ethylene glycol chitosan (205 mg, molecular weight 5000) and 4-isosulfuric acid phenyl-alpha-D-mannoside (9.1 mg) in water, at this time, the structural units of ethylene glycol chitosan and The molar ratio of 4-isosulfuric acid phenyl-alpha-D-mannoside is 1:0.03, after adding water, the concentration of ethylene glycol chitosan is 0.5mg / ml, stirred and reacted at room temperature for 24h, and dialyzed against water, Freeze-dried to obtain mannose-modified ethylene glycol chitosan, and the product obtained was denoted as MAN-GCTS, and its NMR characterization results were compared with those of figure 1 unanimous. It can be seen that MAN has been successfully introduced into GCTS.
[0040] Step 2, the preparation of nano vaccine:
[0041] GPC-3 144-152 (1.5 mg) was dissolved in 1 mL of phosphate-buffered saline (PBS, pH = 7.4); the MAN-GCTS (10 mg) prepared in Step 1 was dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com