Hyaluronic acid-g-folate amphiphilic polymer and its application
A technology of amphiphilic polymers and hyaluronic acid, which is applied in the direction of drug combinations, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problem of poor biocompatibility and biodegradability, and limit single target To solve the problems of nano-drug efficacy and nano-drug structure size, etc., to prolong the circulation time in the body, overcome the poor selectivity of tumor cells, and facilitate long-term stable storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
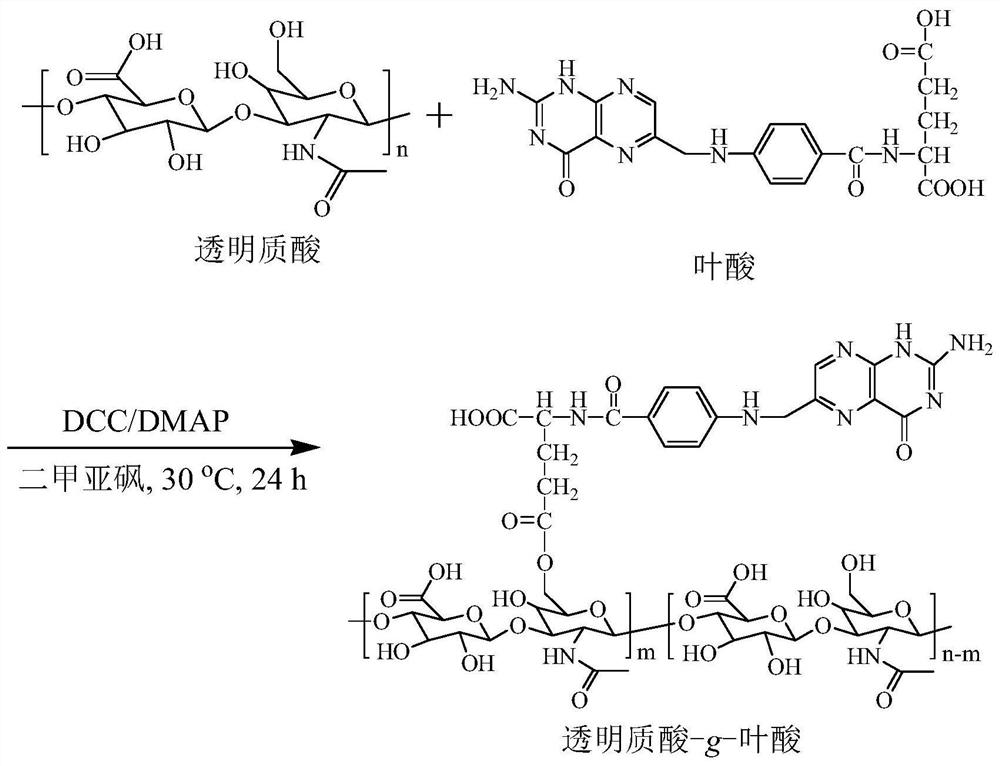
[0040] Example 1 Synthetic polymer hyaluronic acid-folic acid (HA- g -FA) ( M nHA = 35 kDa, DS = 8.5%)
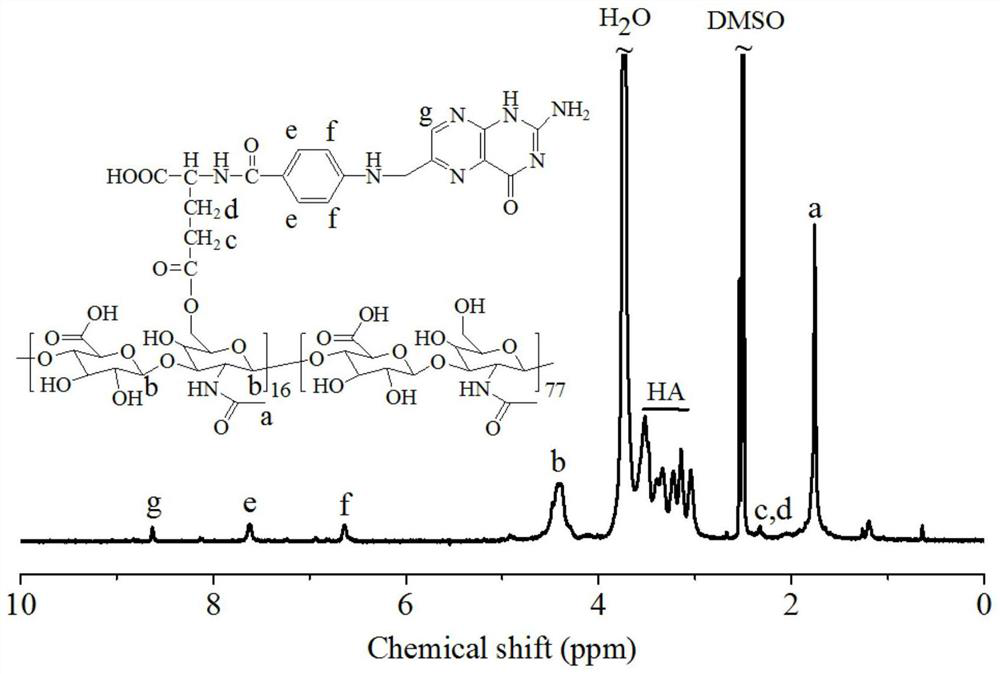
[0041] figure 1 For the example polymer HA- g -Synthetic route map of FA. To a solution of folic acid (FA, 175 mg, 0.40 mmol) in anhydrous dimethylsulfoxide (DMSO, 5 mL) was added 1.5 mL of N,N'-dicyclohexylcarbodiimide (DCC, 163 mg, 0.79 mmol) in DMSO, stirred at 30°C for 12 hours, then added 1 mL 4-dimethylaminopyridine (DMAP, 97 mg, 0.79 mmol) and 4 mL hyaluronic acid (HA, 200 mg, 0.53 mmol -CH 2 OH) in anhydrous DMSO solution at 30°C for 24 hours. After the reaction, hyaluronic acid-g-folate (HA-g-FA) polymer was obtained by suction filtration, dialysis, and freeze-drying, with a yield of 93%. NMR see figure 2 , 1 H NMR (D 2 O: DMSO- d 6 ): Hyaluronic acid (HA): δ (ppm) 1.86–2.01, 3.28–4.02, 4.21–4.75; Folic acid (FA): δ (ppm) 6.64, 7.63, 8.61. NMR results show that its structure is hyaluronic acid- g -Folic acid (HA- g -FA), the degree of substitution...
Embodiment 2
[0042] Example two Synthetic polymer HA- g -FA ( M nHA = 35 kDa, DS = 6.4%)
[0043] Under nitrogen atmosphere, add 1 mL of DCC (109 mg, 0.53 mmol) in anhydrous DMSO (3 mL) solution of folic acid (FA, 116 mg, 0.26 mmol), stir at 30°C for 12 hours, then Add 1 mL DMAP (64 mg, 0.53 mmol) and 4 mL hyaluronic acid (HA, 200 mg, 0.53 mmol) sequentially 2 OH) in DMSO and reacted at 30°C for 24 hours. After the reaction, hyaluronic acid- g - Folic acid (HA-g-FA) polymer in 93% yield. NMR results show that its structure is HA- g -FA, wherein the degree of substitution (DS) of FA is 6.4%.
Embodiment 3
[0044] Example three synthetic polymer HA- g -FA ( M nHA = 35 kDa, DS = 11.1%)
[0045] Under a nitrogen atmosphere, 2 mL of DCC (218 mg, 1.06 mmol) in anhydrous DMSO (6 mL) was added to a solution of folic acid (FA, 232 mg, 0.52 mmol) in anhydrous DMSO, stirred at 30°C for 12 hours, and then Add 1 mL DMAP (128 mg, 1.06 mmol) and 4 mL hyaluronic acid (HA, 200 mg, 0.53 mmol -CH 2 OH) in anhydrous DMSO solution at 30°C for 24 hours. After the reaction, hyaluronic acid- g -Folic acid (HA- g -FA) polymer in 93% yield. NMR results show that its structure is HA- g -FA, wherein the degree of substitution (DS) of folic acid is 11.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
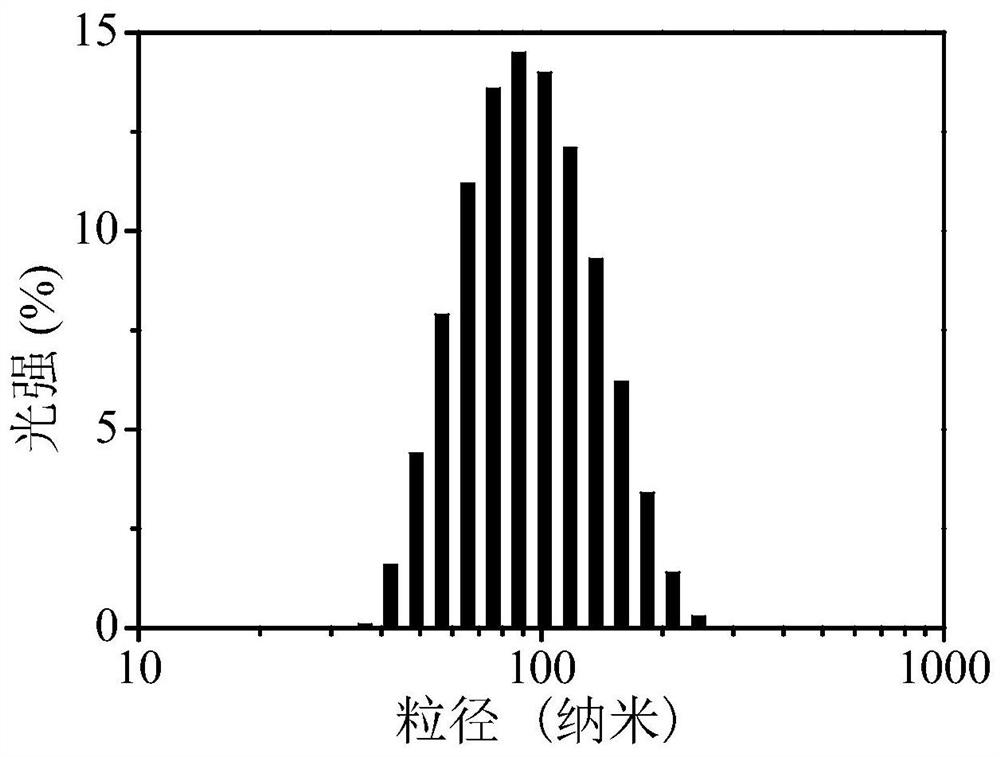
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com