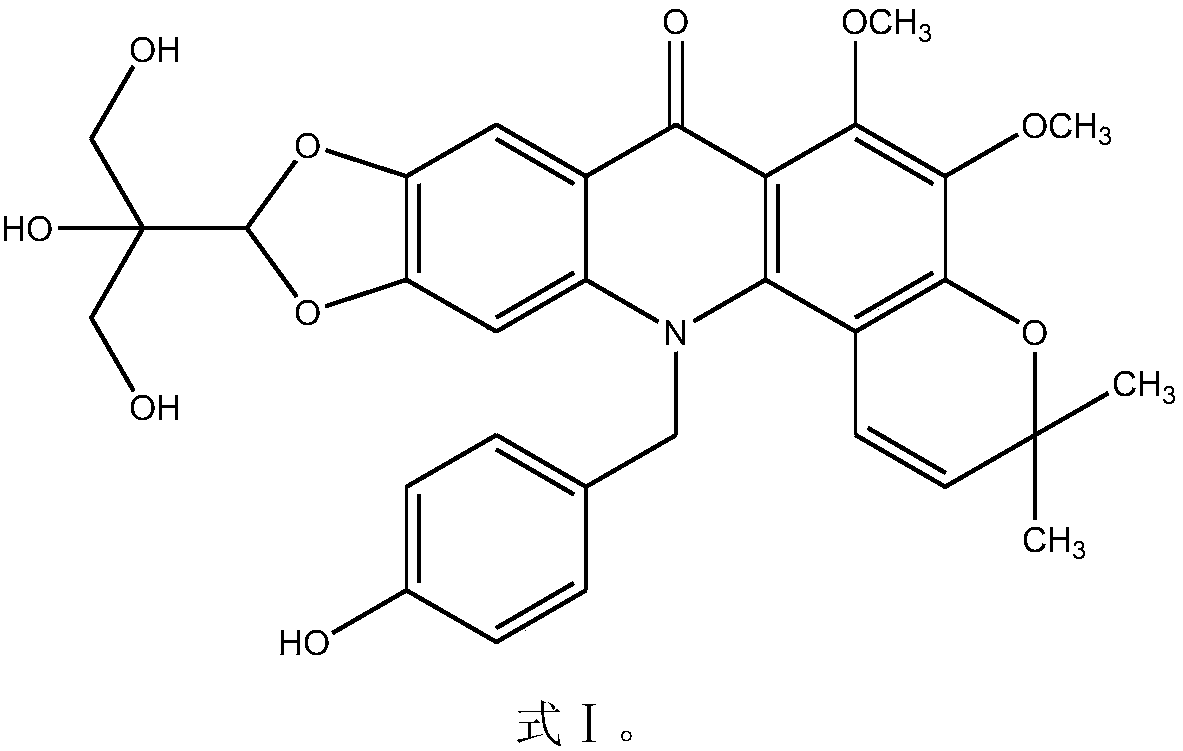
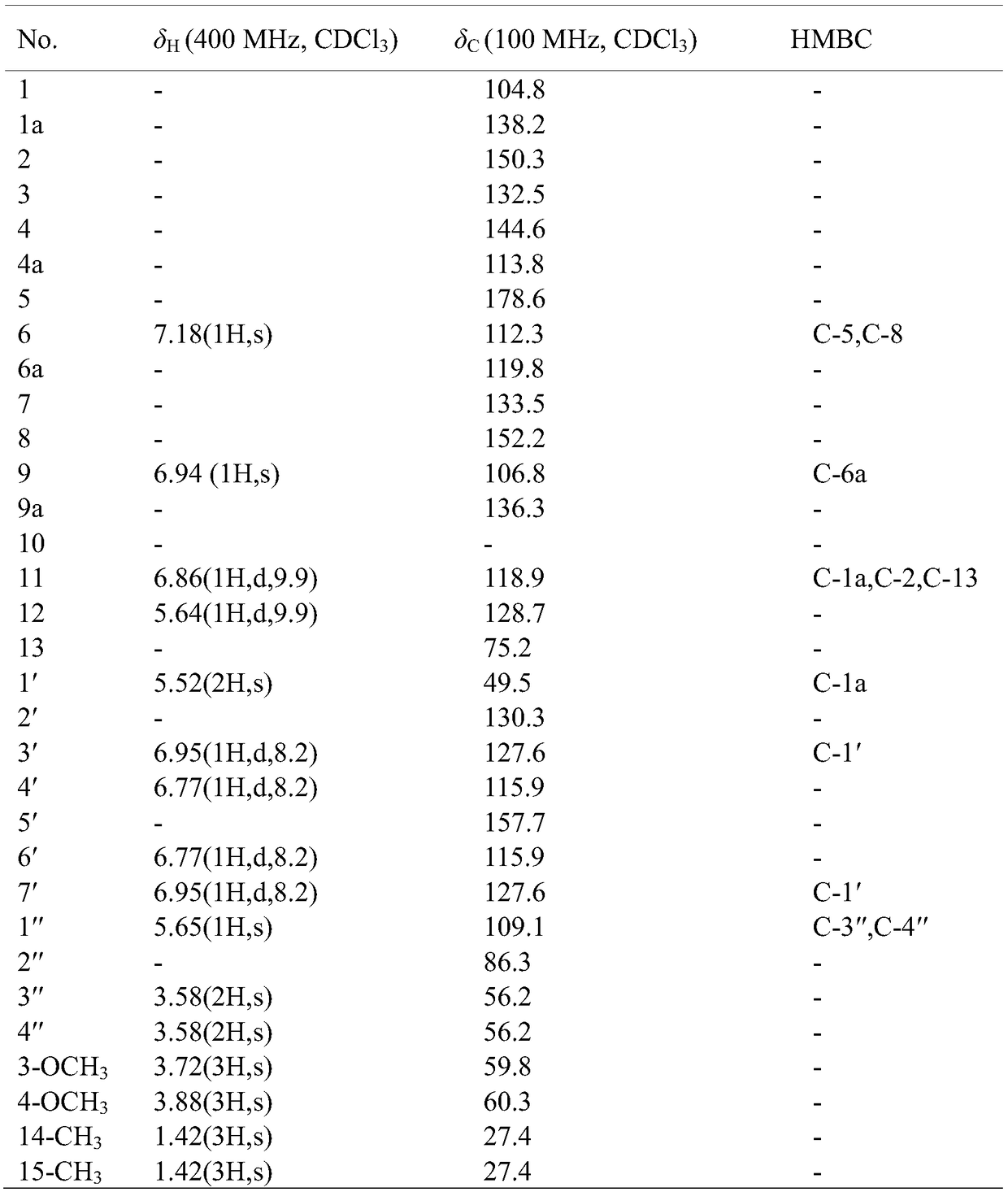
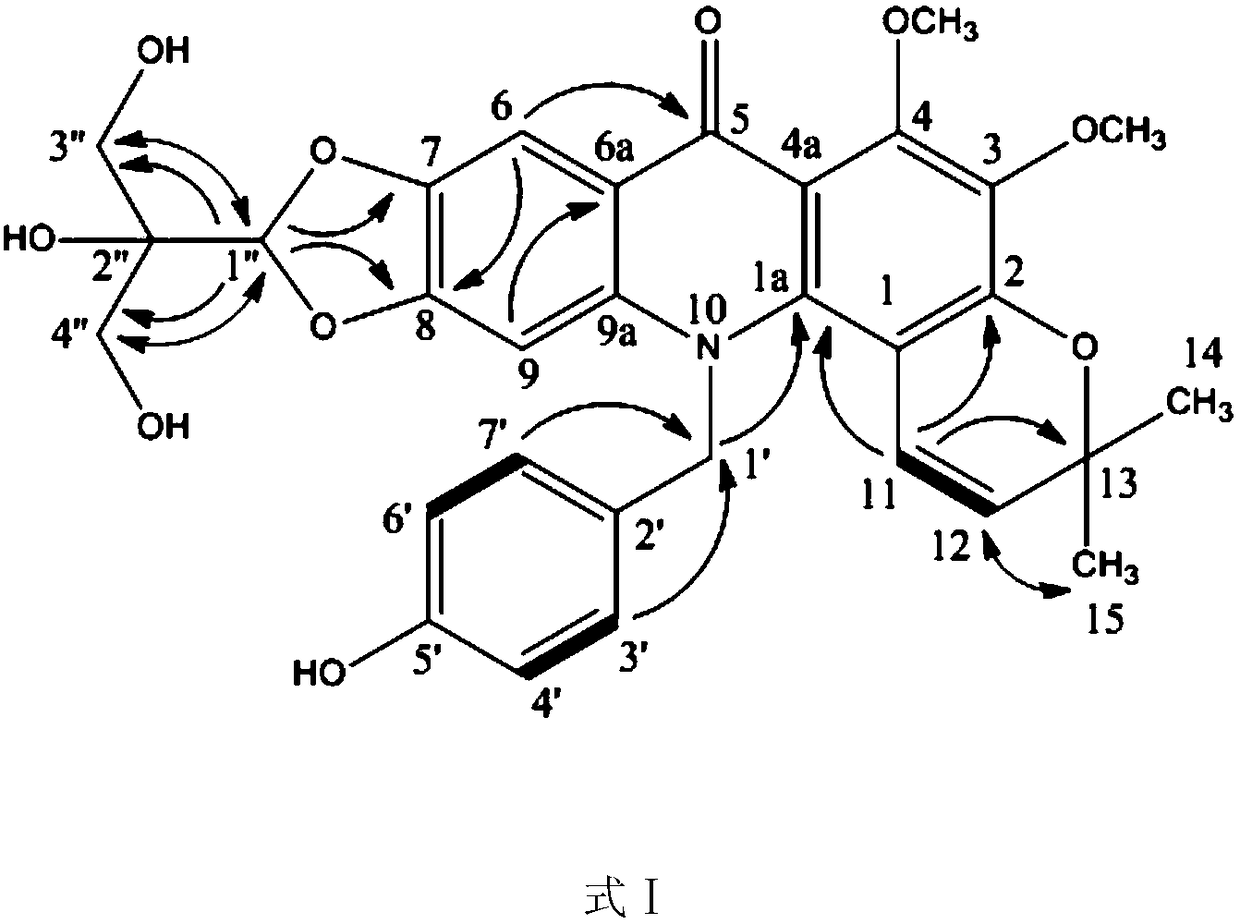
New N-benzyl acridone alkaloid as well as preparation method and use thereof
A technology of benzylacridone type and alkaloids, which is applied in the field of medicine, can solve the problems such as no research reports on fungus, and achieve the effects of being convenient for pharmacological and clinical research, simple and easy to prepare, and novel in structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1N-benzylacridone type alkaloids
[0029] Step (1), take the dried fungus (9 kg) and put it in 95% ethanol, immerse the medicinal material for 2-4 cm, soak at room temperature for 18 hours; then heat and reflux for 3 hours, stand and filter, and concentrate the obtained filtrate to no alcohol taste, to obtain the total thick paste of fungus (850 grams);
[0030] Step (2), according to the volume ratio of 1:1.5, uniformly suspend the total thick paste of fungus in 1.3% HCl solution, let it stand for 22 hours, and filter to obtain the supernatant;
[0031] Step (3), adjust supernatant liquid pH=11 with ammoniacal liquor, extract with chloroform, ethyl acetate, n-butanol successively, each extract is concentrated and dried to obtain chloroform thick paste (36.8 grams), ethyl acetate thick paste (258.5 gram), n-butanol thick ointment (364.0 gram); Adopt DiI-LDL cell phagocytosis test to carry out lipid-lowering activity screening to above-ment...
Embodiment 2
[0041]The hypolipidemic activity of embodiment 2 compound I
[0042] experimental method
[0043] (1) Preparation of DiI-LDL
[0044] DiI (Beijing Zhongsheng Ruitai Technology Co., Ltd.) was dissolved in DMSO to prepare a DiI mother solution of 15 mg / mL. The DiI mother solution was added to the LDL / LPDS (V / V=1:2) mixture, so that the final concentration of DiI was 300 μg / mg LDL. Water bath at 37°C for 18h. The density of the mixture was adjusted to 1.063g / mL with sodium bromide, and ultracentrifuged for 24h. At the top of the gradient solution is labeled LDL, which is separated from free DiI. The upper layer of DiI-LDL was collected, dialyzed for 24 hours, then transferred to PBS buffer (3 L) and dialyzed for another 24 hours. The dialyzed DiI-LDL was filtered and sterilized with a 0.22 μm filter, and its protein content was measured before use.
[0045] (2) Mark HepG2 cells with DiI-LDL
[0046] HepG2 cells (Shanghai Ji Ning Industrial Co., Ltd.) were inoculated on a 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com