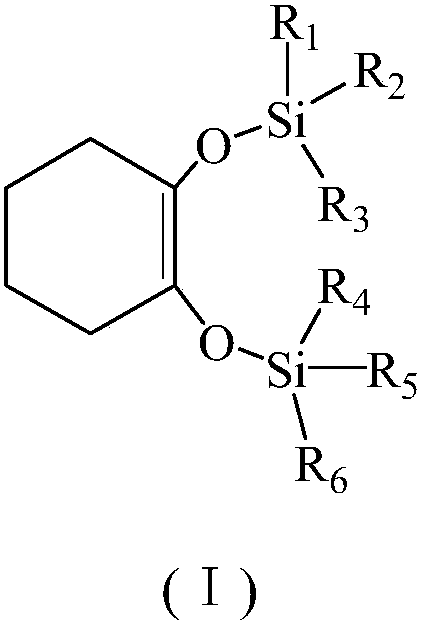
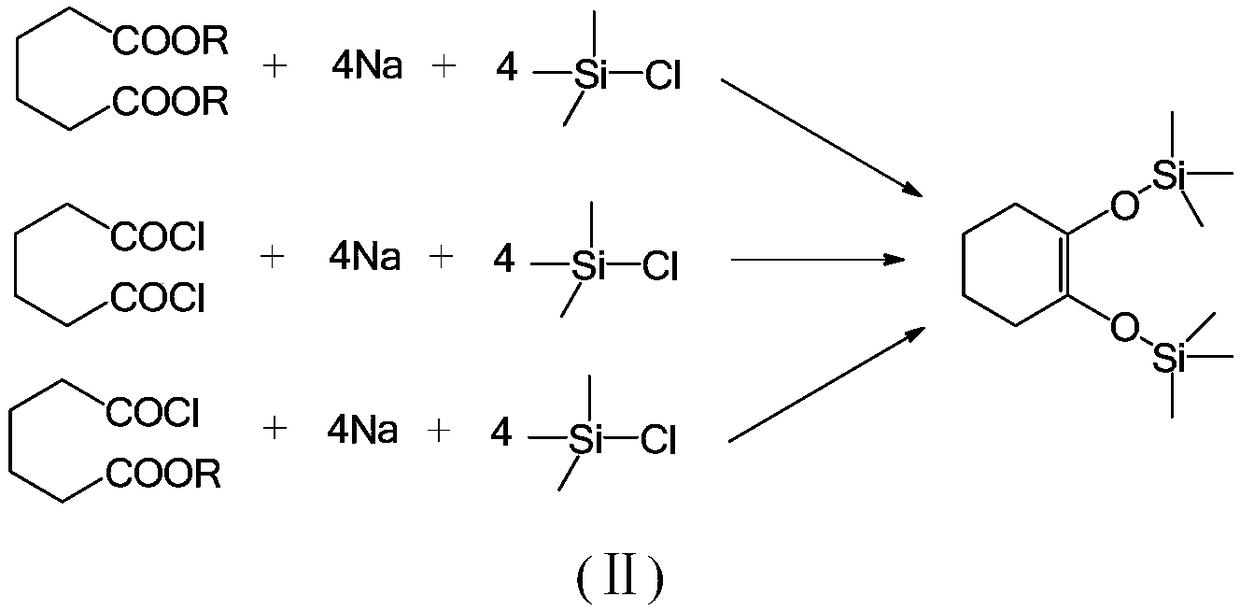
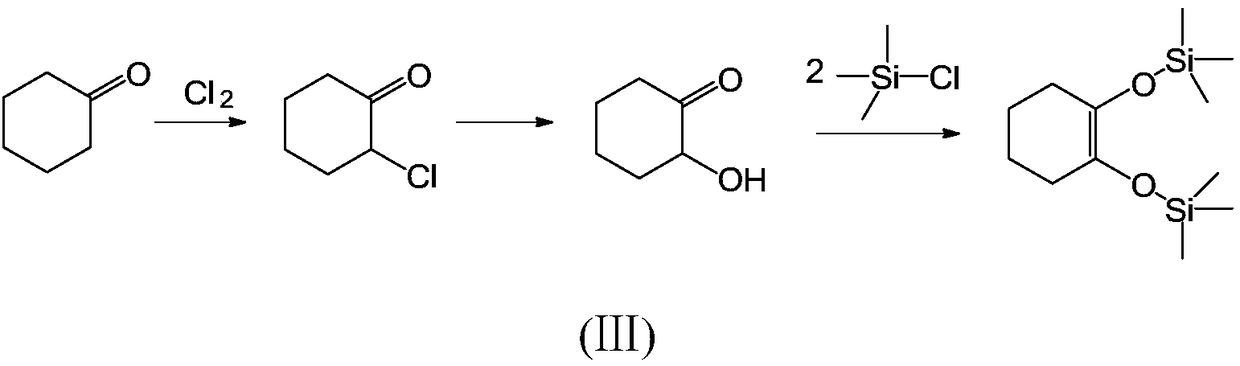
Green synthesis method for 1,2-bi(trialkyl siloxy) cyclohexene
A trialkylsiloxy, green synthesis technology, applied in the periodic table group 4/14 element compounds, silicon organic compounds, chemical instruments and methods, etc. The problem of high temperature of sodium sand can achieve the effect of obvious raw material cost advantage, environmental friendliness and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Synthesis of 1,2-bis(trimethylsiloxy)cyclohexene
[0071] (1) In a four-neck flask with a reflux device, drop into a toluene azeotrope (containing about 30 wt% of silicon ether) containing hexamethyldisiloxane (silicon ether) (25.0 g in pure form, about 0.1539 mol), and heat up Reflux at 100°C to carry out rectification to divide water, and toluene returns to the system. When anhydrous is distilled, add 15.0 g of concentrated sulfuric acid (concentration 98.5%, about 0.1506 mol) dropwise, continue the reaction to carry out rectification and divide water, and toluene returns to the system middle. After 3 hours of reflux reaction, when anhydrous distilled out, GC detected the reaction solution, and when the silicon ether remained <0.5% (GC apparent content), stop the reaction, cool down to room temperature for standby, and simultaneously calculate the bis(trimethyl Silicon) sulfate concentration is about 37.59wt%, about 0.1491mol.
[0072] (2) In a four-necke...
Embodiment 2~10
[0073] Examples 2-10: Synthesis of 1,2-bis(trimethylsiloxy)cyclohexene
[0074] Examples 2-10 were synthesized with reference to the process of Example 1, and the differences in process conditions are shown in Table 1.
[0075] Table 1: Process conditions and synthesis results of Examples 1 to 10
[0076]
[0077]
Embodiment 11
[0078] Example 11: Synthesis of 1,2-bis(triethylsilyloxy)cyclohexene
[0079] (1) In a four-neck flask with a reflux device, drop into a toluene azeotrope (containing about 38.57 wt% of silicon ether) containing hexaethyldisiloxane (silicon ether) (37.68 g in pure form, about 0.1528 mol), Heat up to 120°C and reflux to carry out rectification of water, and toluene is refluxed in the system. in the system. After 3 hours of reflux reaction, when anhydrous distilled out, GC detected the reaction solution, and when the silyl ether remained <0.5% (GC apparent content), the reaction was stopped, and the temperature was lowered to room temperature for use. At the same time, the concentration of bis(triethylsilyl)sulfate was calculated to be about 44.80 wt%, about 0.1490 mol.
[0080] (2) In a four-necked bottle with a reflux device, put in 6.2g (0.1546mol) of calcium powder and 100g of toluene, and add bis(triethylsilicon)sulfate toluene solution dropwise to control the rate of add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Origami | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap