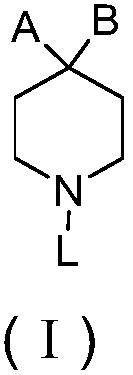
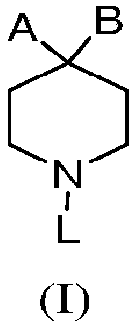
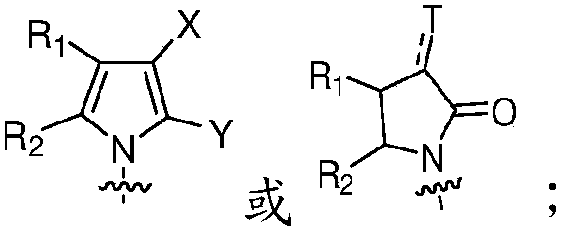
Piperidinyl nociceptin receptor compounds
A technology for compounds and hydrates, applied in the fields of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve the problems of poor duration, short clinical effect, limited treatment options, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0138] Compound preparation
[0139] Those of ordinary skill in the art understand that the piperidinyl nociceptin receptor compound containing formula (I) and the embodiments represented by formula (II), formula (III) and formula (IV) can be synthesized by various synthetic routes . Exemplary syntheses of compounds of formula (II) are shown in Figures 1 to 6 and 10 and described in Examples 1 to 6 and 10 below. Table 1 also provides the compounds of formula II 1 H NMR or TLC data.
[0140] Exemplary syntheses of compounds of formula (III) are shown in Figures 7 and 8 and described in Examples 7 and 8 below. Regarding the compound of formula (III), Table 2 provides the 1 H NMR or TLC data.
[0141] An exemplary route to a compound of formula (IV) is shown in Figure 9 and described in Example 9 below. For compounds of formula (IV), Table 3 provides where indicated 1 H NMR data.
[0142] Composition and method of application
[0143] Compositions provided herein contai...
example 1
[0236] Example 1: 1-(1-((1s,4s)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indole (61) and 1-(1-((1s, Synthesis of 4s)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indole-3-carbaldehyde oxime (81)
[0237] Scheme I depicts this synthesis.
[0238] Option I
[0239]
[0240] Scheme I materials and conditions: a) AcOH, sodium triacetoxyborohydride (STAB), MgSO 4 , DCE, room temperature (general process step A); b) i.TFA, CH 2 Cl 2 , ii.4-isopropyl-cyclohexanone, STAB, AcOH, DCE (general process step B, 2 steps); c) MnO 2 、CH 2 Cl 2 ; d) POCl 3 , DMF; and e) NH 2 OH·HCl, NaOAc·3H 2 O, EtOH:H 2 O(2:1), 110°C.
[0241] General Process Step A: Reductive Amination Using N-Boc-Piperidone : A round bottom flask was charged with aniline substrate (1.00 equiv) and N-Boc-piperidone (1.05 to 1.50 equiv). 1,2-DCE (0.25M) was added, and the mixture was stirred until both components were dissolved. Add MgSO to this solution at ambient temperature 4 (100 wt% of the limiting age...
example 2
[0250] Example 2: Benzyl ((1-(1-((1s,4s)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indol-2-yl)methyl base) carbamate (17) and (1-(1-((1s,4s)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indol-2-yl)methanamine (3) Synthesis
[0251] Scheme II depicts this synthesis.
[0252] Scheme II
[0253]
[0254] Scheme II materials and conditions: a) i.N-Boc piperidone, AcOH, STAB, MgSO 4 , DCE, room temperature (general process step A), ii. TFA, CH 2 Cl 2 , iii. 4-isopropyl-cyclohexanone, STAB, AcOH, DCE (general process step B, 2 steps); b) i. benzyl prop-2-yn-1-ylcarbamate, catalyst PdCl 2 (PPh 3 ) 2 , catalyst cuprous iodide (CuI), DMF: iso-Pr 2 NEt(3:1), ii. Catalyst Cu(OAc) 2 , PhMe, reflux (general process step C, 2 steps); and c) H 2 Airbag, Catalyst 10%Pd / C, NH 3 / MeOH.
[0255] syn-N-(2-iodophenyl)-1-(4-isopropylcyclohexyl)piperidin-4-amine (II-1) :
[0256] i. See General Process Step A. 2-iodoaniline (15.0g, 63.3mmol, 1.00eq), N-Boc-piperidone (18.5g, 95.0mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com