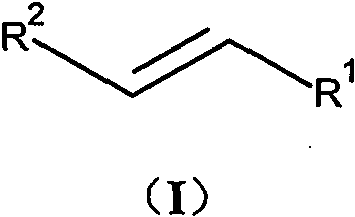
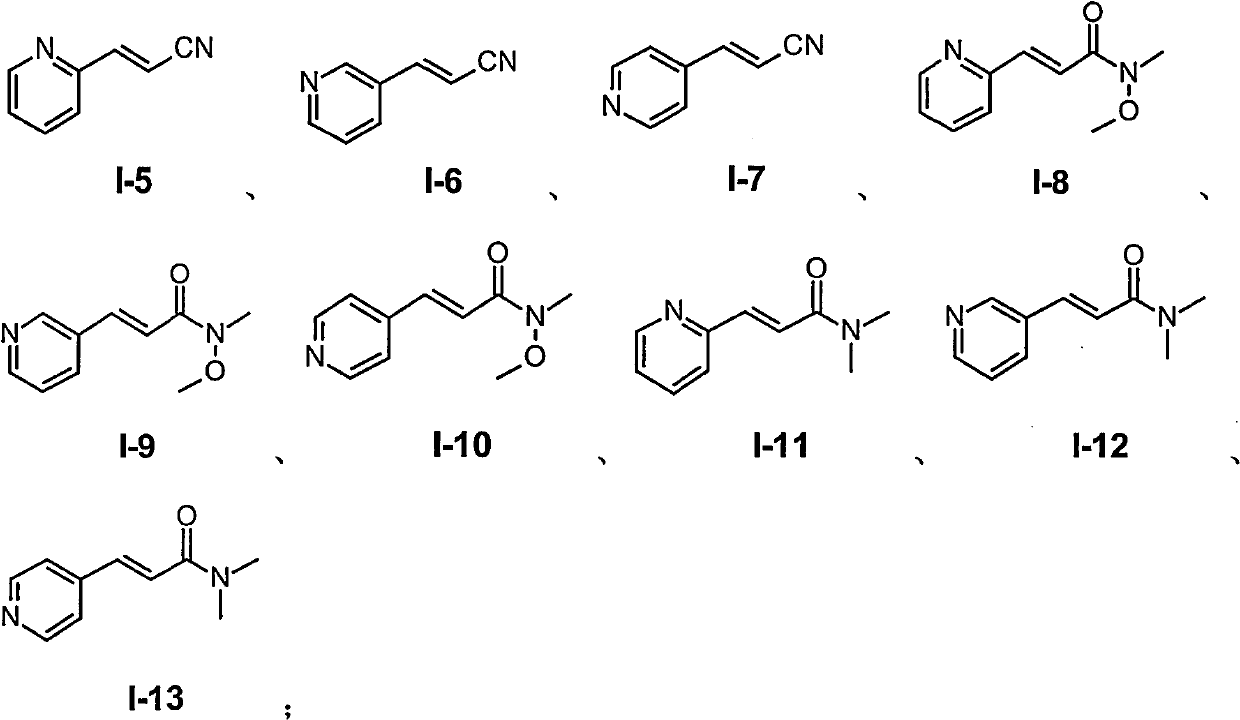
(E)-3-heteroaromatic propyl-2-enoic acid derivative as well as preparation and application thereof
A technology of alkyl and medicine, which is applied in the field of (E)-3-aryl heterocyclyl prop-2-enoic acid derivatives and their preparation and application, and can solve any problems such as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
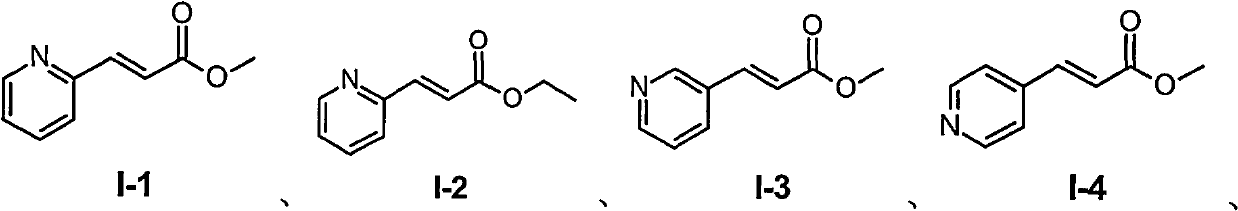
[0047] Steps: Put 0.298 g (2 mmol) of pyridine-2-acrylic acid (compound A) and 10 ml of methanol into the reaction flask, slowly add 0.03 g (0.3 mmol) of concentrated sulfuric acid dropwise, react at room temperature for 3 hours, add dropwise saturated carbonic acid Sodium hydrogen solution, adjust the pH to about 8, then extract with dichloromethane, dry over anhydrous magnesium sulfate, filter, concentrate, and dry to obtain 0.223 g of compound I-1, yield: 68%. 1 H NMR (CDCl 3 ): δ3.82(s, 3H), 6.95(d, 1H, J=16.0Hz), 7.28(m, 1H), 7.43(d, 1H, J=8.0Hz), 7.70(d, 1H, J= 16.0Hz), 7.73(m, 1H), 8.66(d, 1H, J=4.0Hz); MS(ESI): m / z 164[M+H] + .
Embodiment 2
[0049]
[0050] Steps: Put 0.298 g (2 mmol) of pyridine-2-acrylic acid (compound A) and 10 ml of ethanol into the reaction flask, slowly add 0.03 g (0.3 mmol) of concentrated sulfuric acid dropwise, react at room temperature for 3 hours, add saturated carbonic acid dropwise Sodium hydrogen solution, adjust the pH to about 8, then extract with dichloromethane, dry over anhydrous magnesium sulfate, filter, concentrate, and dry to obtain 0.249 g of compound I-2, yield: 72%. 1 H NMR (CDCl 3 ): δ1.34(t, 3H, J=8.0Hz), 4.28(q, 2H, J=8.0Hz), 6.93(d, 1H, J=16.0Hz), 7.28(m, 1H), 7.44(d , 1H, J=8.0Hz), 7.69(d, 1H, J=16.0Hz), 7.73(m, 1H), 8.66(d, 1H, J=4.0Hz); MS(ESI): m / z 178[ M+H] + .
Embodiment 3
[0052]
[0053] Steps: put 0.298 g (2 mmol) of pyridine-3-acrylic acid (compound A) and 10 ml of methanol into the reaction flask, slowly add 0.03 g (0.3 mmol) of concentrated sulfuric acid, react at room temperature for 3 hours, add dropwise saturated carbonic acid Sodium hydrogen solution, adjust the pH to about 8, then extract with dichloromethane, dry over anhydrous magnesium sulfate, filter, concentrate, and dry to obtain 0.233 g of compound I-3, yield: 71%. 1 H NMR (CDCl 3 ): δ3.75 (s, 3H), 6.44 (d, 1H, J = 16.0Hz), 7.27 (dd, 1H, J = 4.0, 8.0Hz), 7.61 (d, 1H, J = 16.0Hz), 7.77 (d, 1H, J=8.0Hz), 8.54(d, 1H, J=4.0Hz), 8.67(s, 1H); MS(ESI): m / z 164[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com