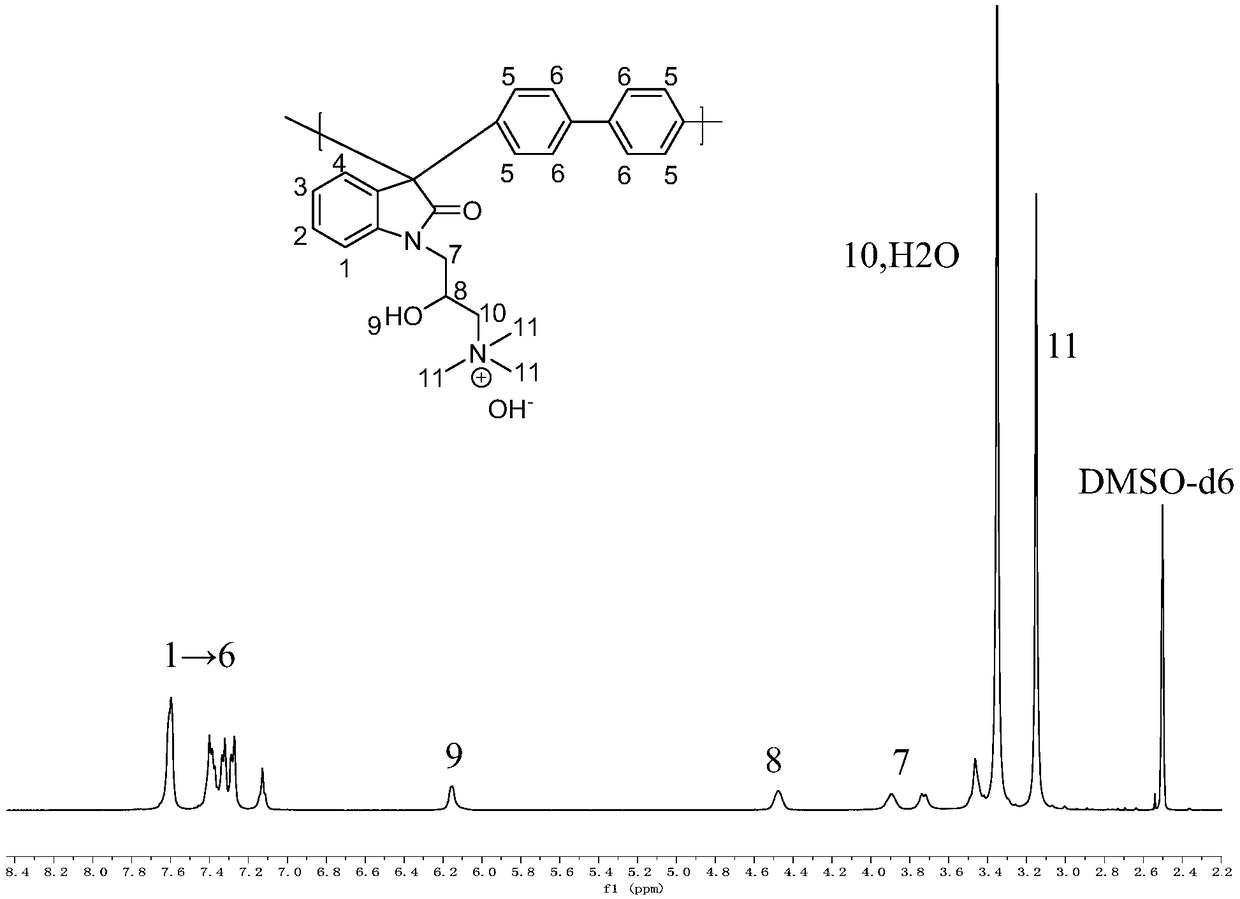
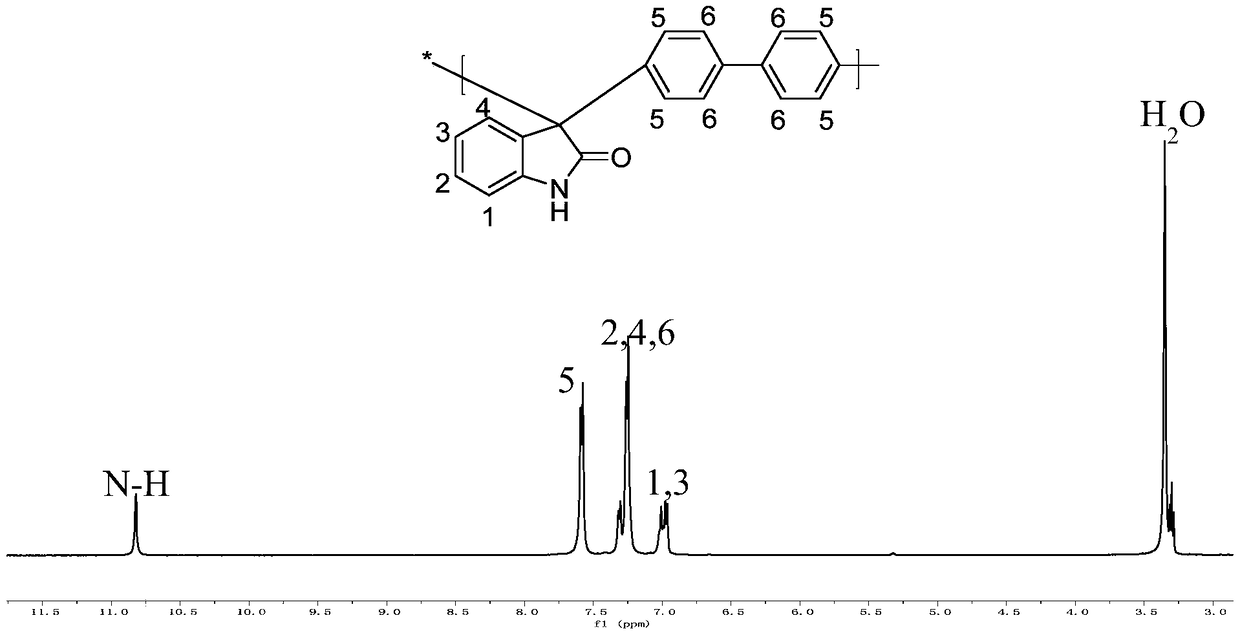
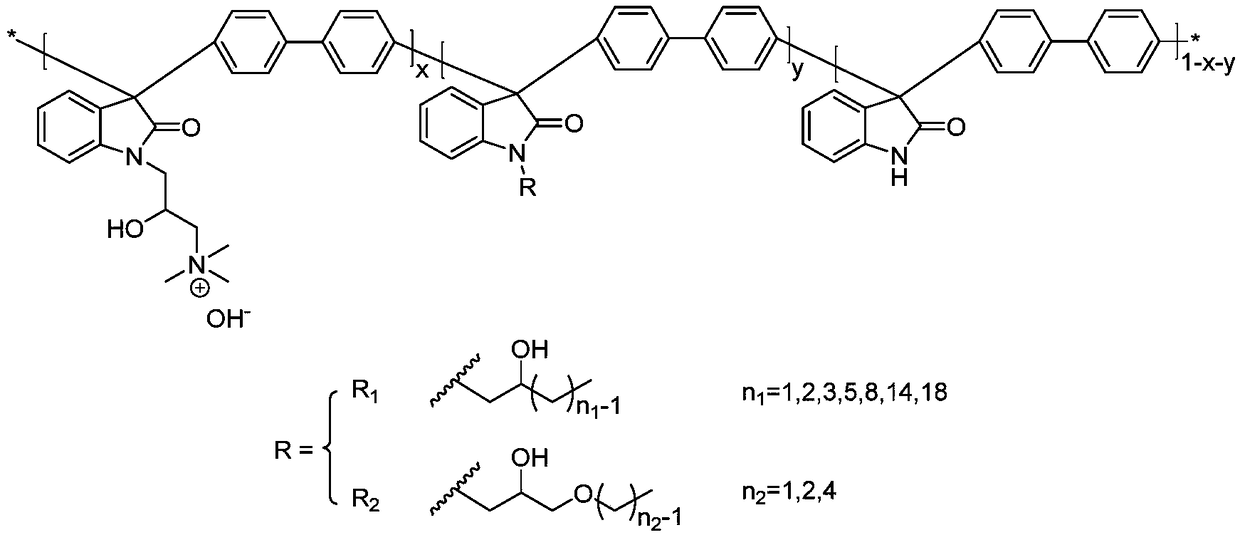
A trimethylamine functionalized polyarylindole anion exchange membrane and a preparation method thereof
An ion exchange membrane, functionalized technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Synthesis of polyarylindole polymer: under the protection of inert gas, 5.25g 2,3-indoledione and 5.42g biphenyl were dissolved in 6mL methylene chloride and 11mL trifluoroacetic acid, after completely dissolving, the The reaction was placed in an ice-bath environment, 25 mL of trifluoromethanesulfonic acid was slowly added, and then the temperature was gradually raised to room temperature for about 0.5 to 2 hours. When the reaction turned into a highly viscous liquid, the reaction ended, and it was poured into methanol to obtain a blocky white solid, soaked for about 12 hours, filtered and dried to obtain a crude product. Dissolve the crude product in about 120mL of NMP, pour it into methanol after completely dissolving to obtain a white fibrous solid, soak for 24 hours, filter, wash with methanol for more than three times, and dry at 60°C for 24 hours in a vacuum environment to obtain the purified polyarylene Indole polymers.
[0046] Preparation of trimethylamine-fu...
Embodiment 2
[0051]Synthesis of polyarylindole polymer: under the protection of inert gas, 5.25g 2,3-indoledione and 5.42g biphenyl were dissolved in 6mL methylene chloride and 11mL trifluoroacetic acid, after completely dissolving, the The reaction was placed in an ice-bath environment, 25 mL of trifluoromethanesulfonic acid was slowly added, and then the temperature was gradually raised to room temperature for about 0.5 to 2 hours. When the reaction turned into a highly viscous liquid, the reaction ended, and it was poured into methanol to obtain a blocky white solid, soaked for about 12 hours, filtered and dried to obtain a crude product. Dissolve the crude product in about 120mL of NMP, pour it into methanol after completely dissolving to obtain a white fibrous solid, soak for 24 hours, filter, wash with methanol for more than three times, and dry at 60°C for 24 hours in a vacuum environment to obtain the purified polyarylene Indole polymers.
[0052] Preparation of trimethylamine-fun...
Embodiment 3
[0057] Synthesis of polyarylindole polymer: under the protection of inert gas, 5.25g 2,3-indoledione and 5.42g biphenyl were dissolved in 6mL methylene chloride and 11mL trifluoroacetic acid, after completely dissolving, the The reaction was placed in an ice-bath environment, 25 mL of trifluoromethanesulfonic acid was slowly added, and then the temperature was gradually raised to room temperature for about 0.5 to 2 hours. When the reaction turned into a highly viscous liquid, the reaction ended, and it was poured into methanol to obtain a blocky white solid, soaked for about 12 hours, filtered and dried to obtain a crude product. Dissolve the crude product in about 120mL of NMP, pour it into methanol after completely dissolving to obtain a white fibrous solid, soak for 24 hours, filter, wash with methanol for more than three times, and dry at 60°C for 24 hours in a vacuum environment to obtain the purified polyarylene Indole polymers.
[0058] Preparation of trimethylamine-fu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com