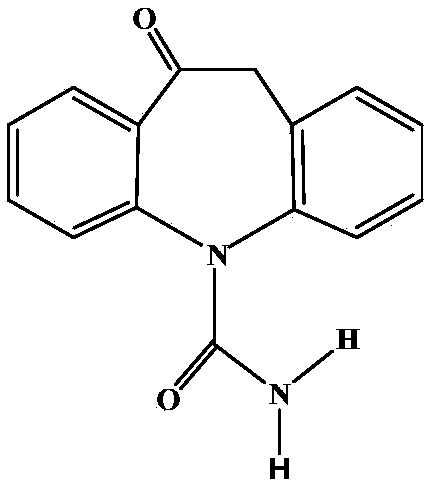
Oxcarbazepine-containing oral dispersing membrane and preparation method thereof
A technology of dispersing film and oral cavity, which is applied in the field of pharmaceutical preparations, can solve the problems of patients' strong psychological suggestion of medication, unfavorable medication privacy, and the impact of treatment effects, etc., and achieve the effect of good taste, convenient medication, and easy acceptance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
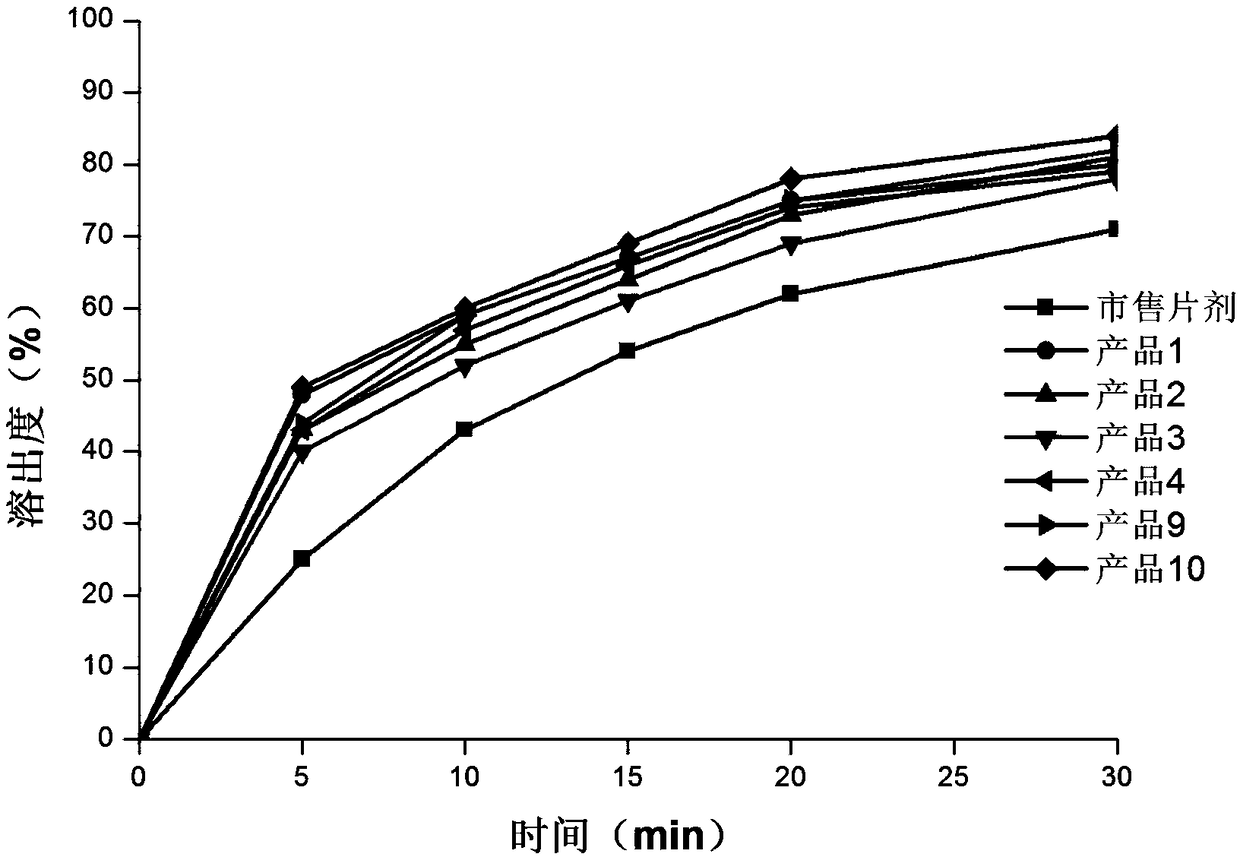
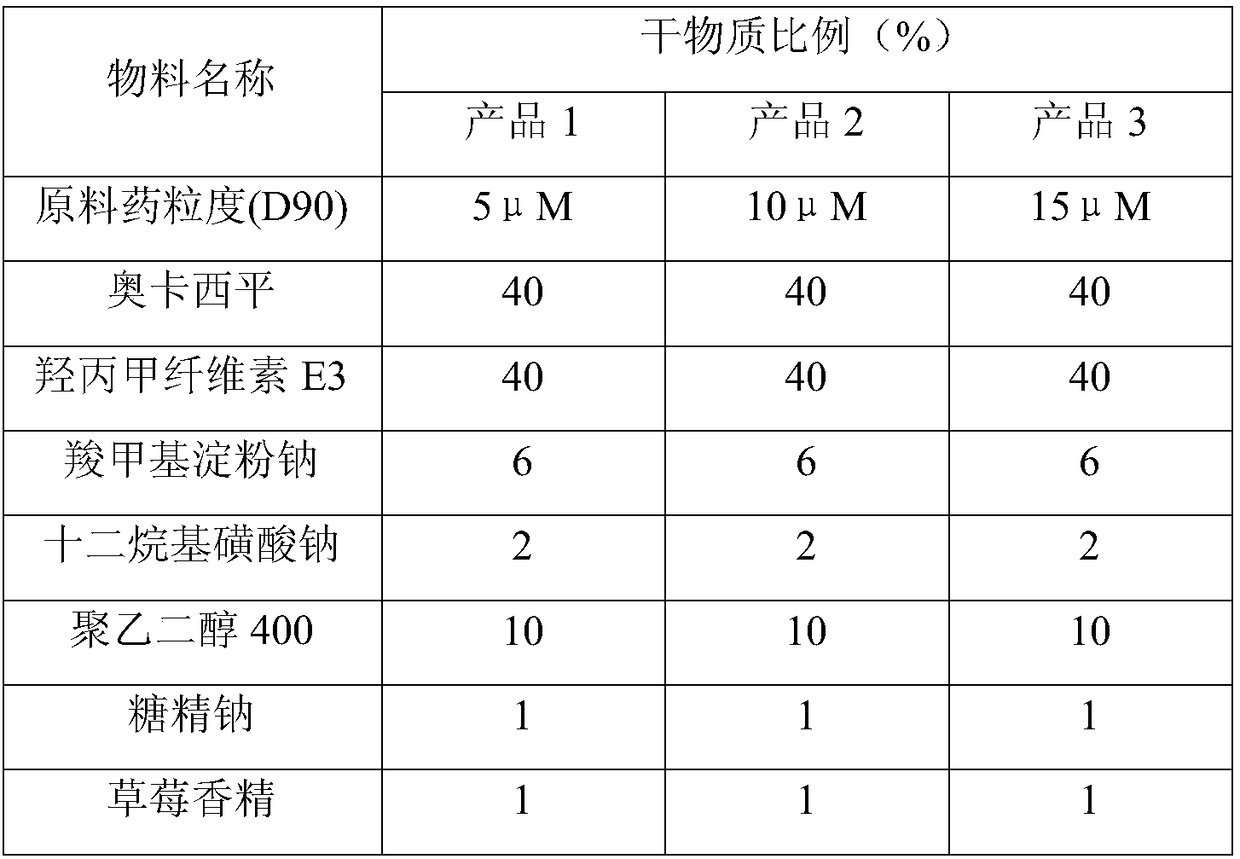
[0048] Influence of API Particle Size on Product Quality
[0049]
[0050] The appearance of the film preparation prepared by the above components is light yellow, complete and smooth, uniform in thickness, the breaking force is in the range of 15-25N, and the toughness is good, which meets the requirements of production, transportation and use. The disintegration time limit is within 15-30 seconds.
Embodiment 2
[0052] Influence of dosage of API and film-forming agent on product quality
[0053]
[0054] The appearance of the film preparation prepared by the above components is light yellow, complete and smooth, uniform in thickness, the tensile force is in the range of 16-28N, and the toughness is good, which meets the requirements of production, transportation and use. The disintegration time limits are all within 25-40 seconds.
Embodiment 3
[0056] Influence of other material proportions on product quality
[0057]
[0058] The appearance of the film preparation prepared by the above components is light yellow, complete and smooth, with uniform thickness, the tensile force is in the range of 12-34N, and the toughness is good, which meets the requirements of production, transportation and use. The disintegration time limits are all within 20-49 seconds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Breaking force | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com