Patents
Literature
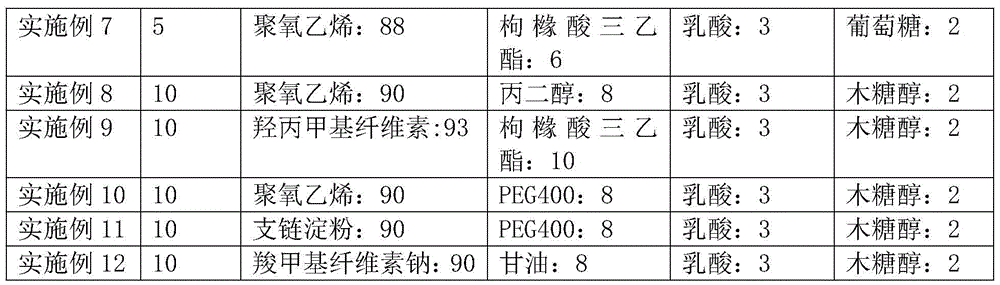
32 results about "Orodispersible film" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A personalised orodispersible film can be printed on-site by the pharmacist in order to respond to the needs of the individual patient (Alomari et al., 2014, Lind et al., 2016). For instance, the required therapeutic dose can be adjusted on-site in particular benefitting paediatric patients.
Oral dispersible films
ActiveUS20150038594A1Good wetting and spreadability propertySuitable mechanical propertyPharmaceutical delivery mechanismPharmaceutical non-active ingredientsHydrophobic polymerPlasticizer
Described herein are orodispersible films comprising a film forming hydrophobic polymer, a disintegrant, a plasticizer and a stabilizer.
Owner:BLUEPHARMA
Desloratadine oral dispersible film
InactiveCN102940617ADisintegrates quicklyQuick effectOrganic active ingredientsImmunological disordersAllergic dermatitisIrritation
The invention relates to a desloratadine oral dispersible film which comprises desloratadine, biologically-acceptable water-soluble polymers, plasticizers, purified water and the like, can be dispersed or dissolved quickly in the oral cavity, and is used for treating allergic rhinitis, allergic rhinitis and asthma syndrome, allergic nasal conjunctivitis, allergic dermatitis, allergic asthma and the like. The desloratadine oral dispersible film is free of irritation, convenient to take and carry, fast in effect, good in taste and particularly suitable for being used by the olds and children.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Oxiracetam oral dispersion membrane preparation and preparation method thereof
InactiveCN106822057AMedication convenienceNot easy to spit outOrganic active ingredientsNervous disorderSide effectBioavailability
The invention relates to an oxiracetam oral dispersion membrane preparation and a preparation method thereof. The oxiracetam oral dispersion membrane preparation is prepared from a composite membrane forming material, a plasticizing agent, a filling agent and the like. The oxiracetam oral dispersion membrane preparation has the advantages that the preparation can be dissolved by less saliva in an oral cavity, and can be taken without using water, so that the taking is convenient; the easiness in vomiting is avoided after sticking onto a tongue, and the Oxiracetam oral preparation is suitable for patients with difficulty in swallowing; by utilizing mucosae to absorb, the first pass elimination effect is avoided, the bioavailability is improved, the medicine dosage is reduced, and the side effect is decreased. The preparation method has the advantages that the preparation technology is simple, and the preparation method is suitable for industrialized production.
Owner:CHONGQING RUNZE PHARM CO LTD
Ibuprofen oral dispersing film agent
InactiveCN102935078AReduce volumeReduce dosageOrganic active ingredientsAntipyreticPlasticizerPurified water
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Orodispersible films having quick dissolution times for therapeutic and food use
InactiveUS20170143623A1Avoid brittlenessFast dissolution timeOrganic active ingredientsPharmaceutical non-active ingredientsMedicinePlasticizer
The present invention concerns an orodispersible self-supporting film free from hydrocolloids comprising:a) a film-forming substance consisting of a maltodextrin in an amount comprised between 40 and 80% by weight;b) one or more plasticizer in a total amount comprised between 15 and 55% by weight;e) a surfactant System in an amount comprised between 0.5 and 6% by weight;d) an active ingredient for food or therapeutic use in an amount between 0.05 and 30% by weight,said orodispersible self-supporting film free from hydrocolloids further containing a homopolymer or a copolymer of vinyl acetate in a quantity comprised between 1 and 20% by weight where the percentages are calculated on the total weight of said film.
Owner:PHARMAFILM
Orodispersible film composition comprising enalapril for the treatment of hypertension in a pediatric population
InactiveUS20170165315A1Reduce the amount requiredThe process is stable and efficientOrganic active ingredientsDipeptide ingredientsPharmacyPediatric population
The present invention relates to an oral applicable therapeutic dosage form, in particular an orodispersible film comprising Enalapril or pharmaceutically acceptable salts thereof for use in the treatment of hypertension in a pediatric population. The pediatric population is defined from 1 to 18 years of age. The present invention also provides a method of manufacturing of such a dosage form.
Owner:PHARMATHEN
Orodispersible film composition comprising enalapril for the treatment of hypertension in a pediatric population
ActiveUS20200360461A1Reduce the amount requiredProcess stabilityOrganic active ingredientsDipeptide ingredientsPharmaceutical medicinePediatric population
The present invention relates to an oral applicable therapeutic dosage form, in particular an orodispersible film comprising Enalapril or pharmaceutically acceptable salts thereof for use in the treatment of hypertension in a pediatric population. The pediatric population is defined from 1 to 18 years of age. The present invention also provides a method of manufacturing of such a dosage form.
Owner:PHARMATHEN
Oral dispersible film composition
PendingUS20190336453A1Reduce in quantityProcess greenerCosmetic preparationsToilet preparationsChemical compositionMedicine
Pharmaceutical and nutraceutical composition in the form of oral dispersible films (ODFs) using twin-screw hot melt extrusion was described. In the present disclosure, there is provided an oral dispersible film composition comprising: (a) maltodextrin; and (b) hydroxypropyl cellulose, wherein the weight ratio of maltodextrin to hydroxypropyl cellulose is in the range of 1:1-3:1. The prepared films of pharmaceutical and nutraceutical composition are uniform in film thickness, have excellent physical attributes and can be directly packed after cutting.
Owner:JUBELN LIFESCI PVT LTD
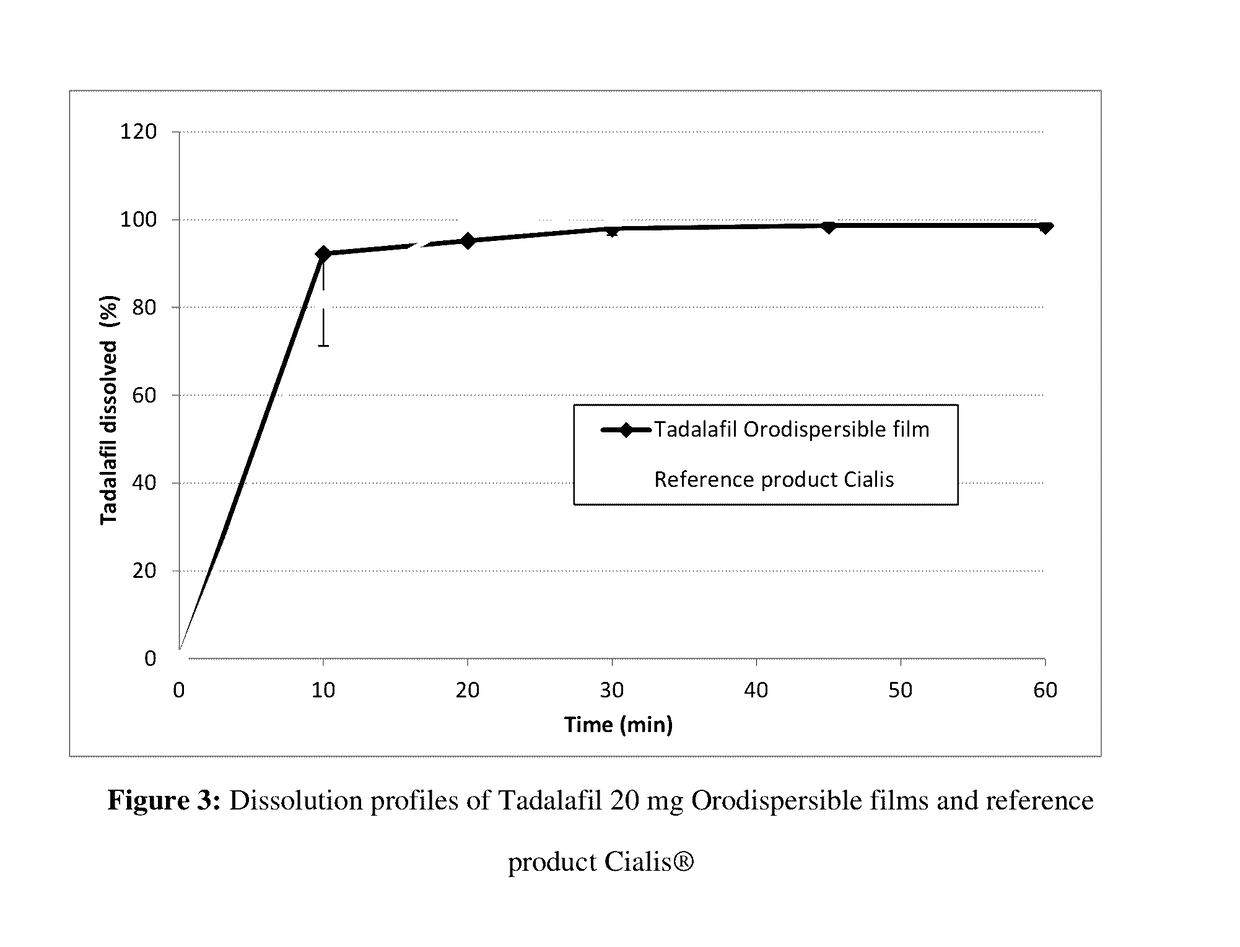
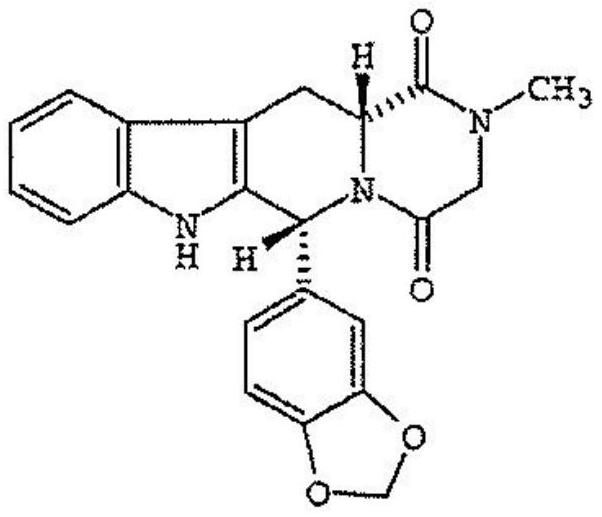
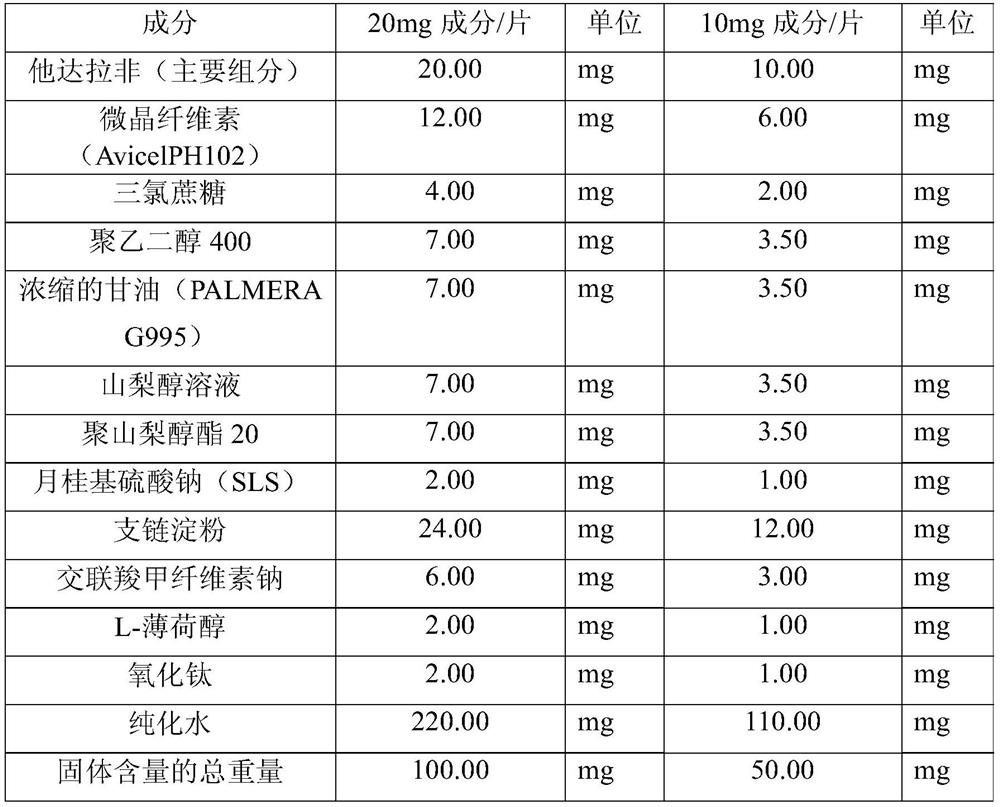
Tadalafil oral dispersible film and preparing method thereof
ActiveCN107106508AAvoid breakingHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsPEG 400Plasticizer
The present disclosure relates to compositions for preparing an oral dispersible film comprising tadalafil or its pharmaceutically acceptable salt thereof, surfactant, plasticizer and sweetening agent; an oral dispersible film; and methods of preparing thereof. The oral dispersible film of the present disclosure can improve dissolution rate of the tadalafil without complicated processes and has superior stability compared to previous Cialis because generation of related compounds are inhibited regardless of existence of a packaging. Additionally, superior film properties comprising prevention of film breakage, securement of flexibility and prevention of oil leakage from film formulation can be maintained when comprising specific content of a polyethylene glycol 400 as the plasticizers.
Owner:SEOUL PHARMA
Oral dissolving film preparation containing bulleyaconitine A and preparation technology of oral dissolving film preparation
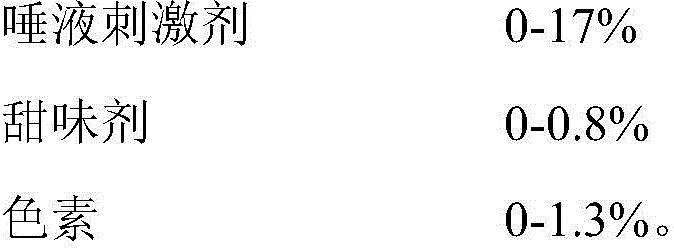
The invention belongs to the field of medicinal preparations, and discloses an oral dissolving film preparation containing bulleyaconitine A and a preparation technology of the oral dissolving film preparation. The dissolving film contains bulleyaconitine A, polyacrylic resin II, a film forming substrate, a sweetening agent, a saliva irritant and essence. With the dissolving film, the tingling sensation and bitter taste of bulleyaconitine A can be well masked, and the preparation method is simple and feasible.
Owner:YUNNAN INST OF MATERIA MEDICA
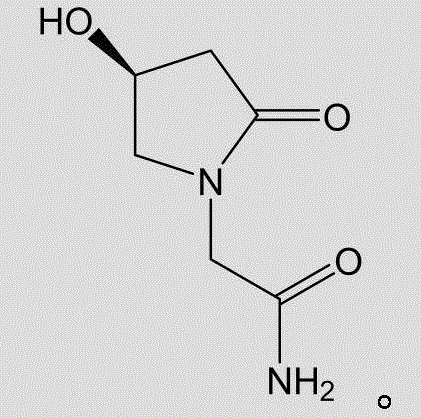
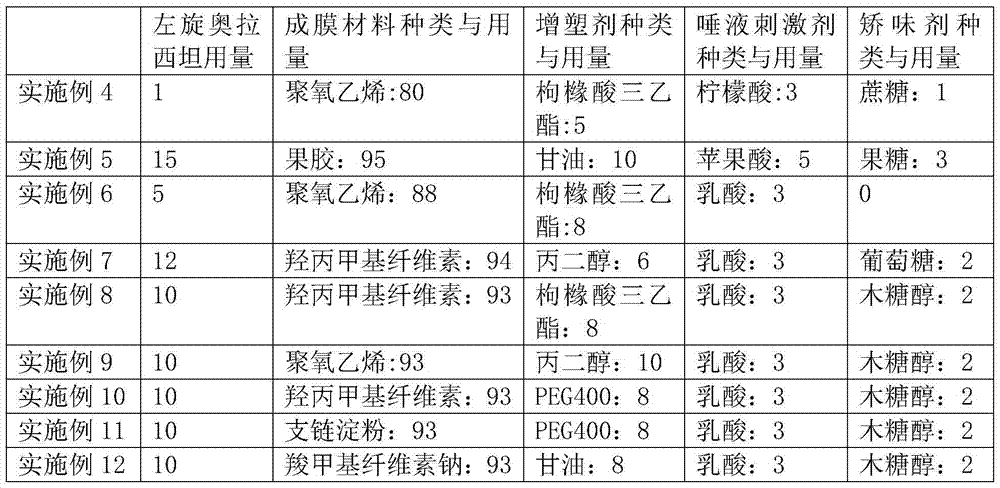
Method for preparing levorotatory oxiracetam oral dispersible film preparation
InactiveCN106821957ASolve the prone to softnessSolve the problem of poor film formationOrganic active ingredientsNervous disorderMedicinePlasticizer
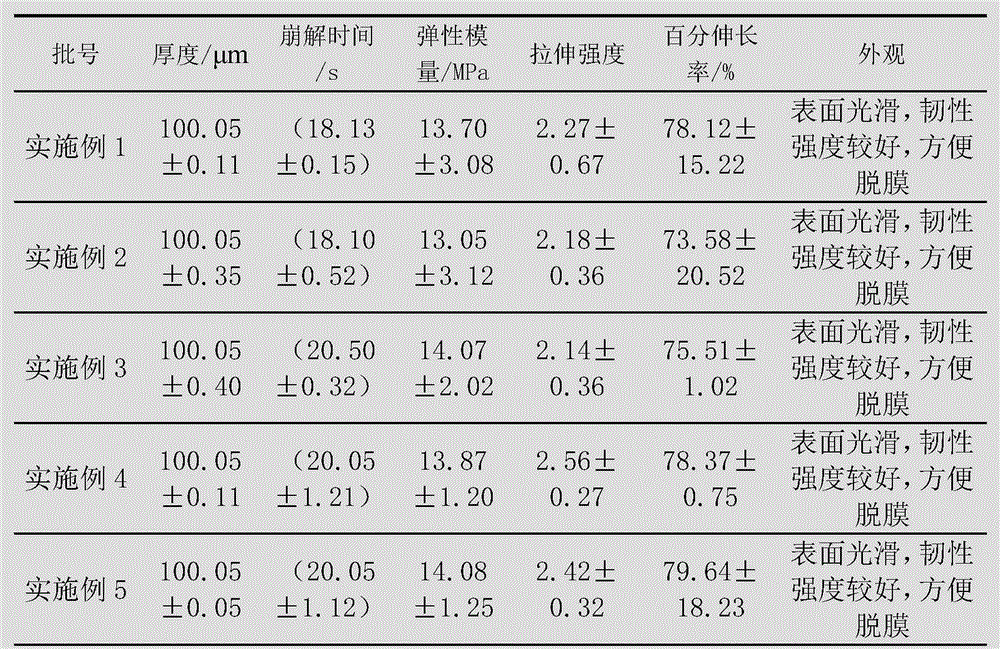
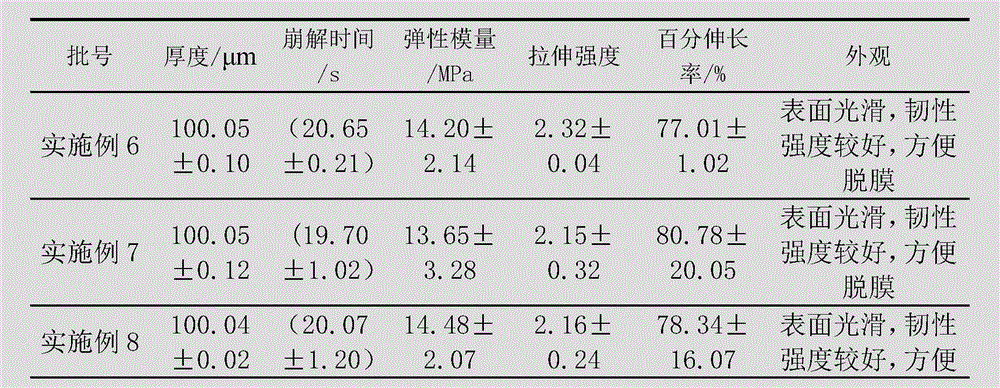
The invention discloses a method for preparing a levorotatory oxiracetam oral dispersible film preparation. The levorotatory oxiracetam oral dispersible film preparation is made by using a film forming material, a plasticizer, a filling agent, a saliva irritant, correctant and levorotatory oxiracetam as raw materials and through coating, drying and stripping by using a film coating and drying machine for a medicinal film. According to the method, the levorotatory oxiracetam oral dispersible film preparation is prepared by using a film coating machine for the medicinal film; the thickness, the coating speed and the drying temperature of the oral dispersible film preparation are strictly controlled; thus, the process is stabilized; the quality of a product is guaranteed; aspects of the brittlement, the disintegration time limit, the dissolution time and the like of the levorotatory oxiracetam oral dispersible film are enabled to be more beneficial to clinical application.
Owner:CHONGQING RUNZE PHARM CO LTD
Orodispersible films for the manufacturing of individualised medicine or for large scale production
InactiveUS20140186427A1Increases flexibility and plasticity and fluidityBiocideOrganic active ingredientsActive agentPersonalized medicine
The present invention pertains to oral applicable therapeutic dosage forms, in particular to orodispersible films. The present invention especially is directed to orodispersible films comprising a base layer substantially free of therapeutically active agents and a top layer comprising the desired therapeutically active agents. The present invention also concerns suitable base layers for such orodispersible films as well as therapeutical uses thereof and methods for manufacturing them.
Owner:TESA LABTEC
Oral dispersible films
ActiveUS9603935B2Good wetting and spreadability propertySuitable mechanical propertyBiocideHalogenated hydrocarbon active ingredientsPolymer scienceHydrophobic polymer
Described herein are orodispersible films comprising a film forming hydrophobic polymer, a disintegrant, a plasticizer and a stabilizer.
Owner:BLUEPHARMA
L-oxiracetam oral dispersible membrane and preparation method thereof
ActiveCN106822058AMedication convenienceNot easy to spit outOrganic active ingredientsNervous disorderOlder peoplePlasticizer
The invention relates to an L-oxiracetam oral dispersible membrane and a preparation method thereof. The L-oxiracetam oral dispersible membrane is prepared by adopting materials, such as a composite membrane-forming material, a plasticizer and a filler. After being stuck to the tongue, the L-oxiracetam oral dispersible membrane cannot be easily spit out, and therefore is suitable for old people with dysphagia. Moreover, because the L-oxiracetam oral dispersible membrane can be absorbed through the mucous membrane, the first pass elimination effect is prevented, the bioavailability is increased, the dosage of medication is reduced, and thereby the side effect of the medicine is reduced. The preparation process of the invention is simple, and therefore is suitable for industrialized production.
Owner:CHONGQING RUNZE PHARM CO LTD
Dextromethorphan hydrobromide oral dispersible film agent and preparation method thereof
ActiveCN103705493AGreat tasteImprove complianceOrganic active ingredientsPharmaceutical non-active ingredientsDextromethorphan HydrobromidePlasticizer
The invention provides a dextromethorphan hydrobromide oral dispersible film agent. The film agent comprises dextromethorphan hydrobromide, polacrilin or polacrilin potassium with grain diameter of 50-150mu m, a film forming agent, a plasticizer, a disintegrating agent, a wetting agent and water, wherein dextromethorphan hydrobromide and polacrilin or polacrilin potassium form a dextromethorphan hydrobromide compound, and the weight ratio of dextromethorphan hydrobromide to polacrilin or polacrilin potassium is 1:1-1:2, and preferably 1:1.3-1:1.5; the film forming agent comprises hydroxypropyl methylcellulose E3 and hydroxypropyl methylcellulose E50. The invention also provides a preparation method of the dextromethorphan hydrobromide oral dispersible film agent. The dextromethorphan hydrobromide oral dispersible film agent can well solve the problem of poor taste of dextromethorphan hydrobromide, is particularly suitable for children and old patients, and can remarkably improve the compliance of patients. An oral film tablet is nicer by an optimal preparation method.
Owner:山西皇城相府药业股份有限公司
Levo oxiracetam oral cavity dispersing film preparation, and preparation method thereof
InactiveCN106852917AReduce moisture contentGuaranteed uniformityOrganic active ingredientsNervous disorderPlasticizerHot melt
The invention discloses a levo oxiracetam oral cavity dispersing film preparation. The levo oxiracetam oral cavity dispersing film preparation comprises 1 to 15 parts of levo oxiracetam, 85 to 95 parts of a film forming material, 5 to 10 parts of a plasticizer, and 2 to 5 parts of a saliva irritant. According to the preparation method, the above raw materials are subjected to full grinding and uniform mixing, and are delivered into a hot-melt lamination machine for extrusion so as to obtain a finished product. The appearance of the obtained levo oxiracetam oral cavity dispersing film preparation is complete, color is uniform, thickness is uniform, physical and chemical properties are stable, in oral cavity, the levo oxiracetam oral cavity dispersing film preparation can be dissolved with a small amount of saliva, taking of the levo oxiracetam oral cavity dispersing film preparation with water is not necessary, the levo oxiracetam oral cavity dispersing film preparation is convenient to eat, and the preparation method is simple, and is suitable for industrialized production.
Owner:CHONGQING RUNZE PHARM CO LTD
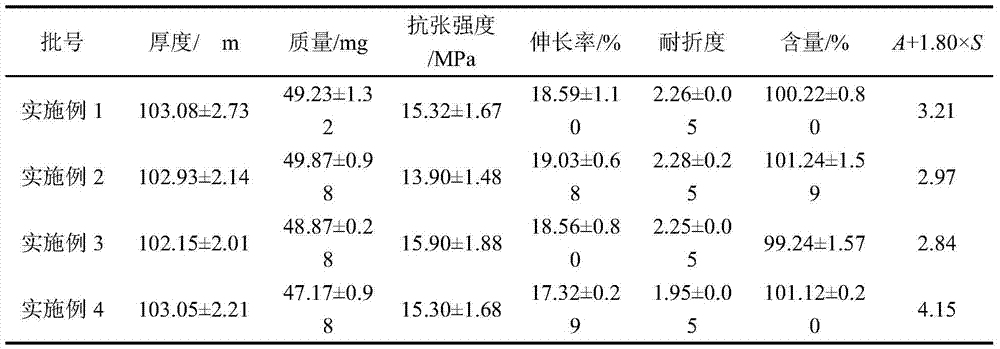
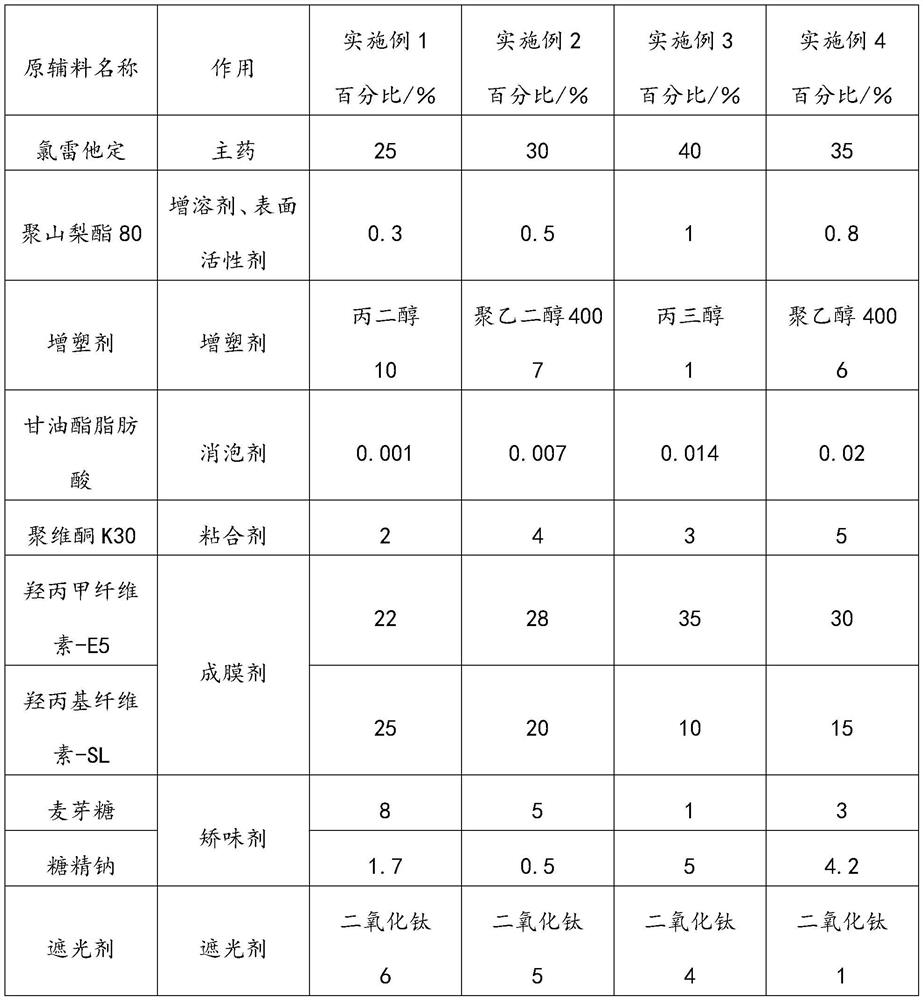
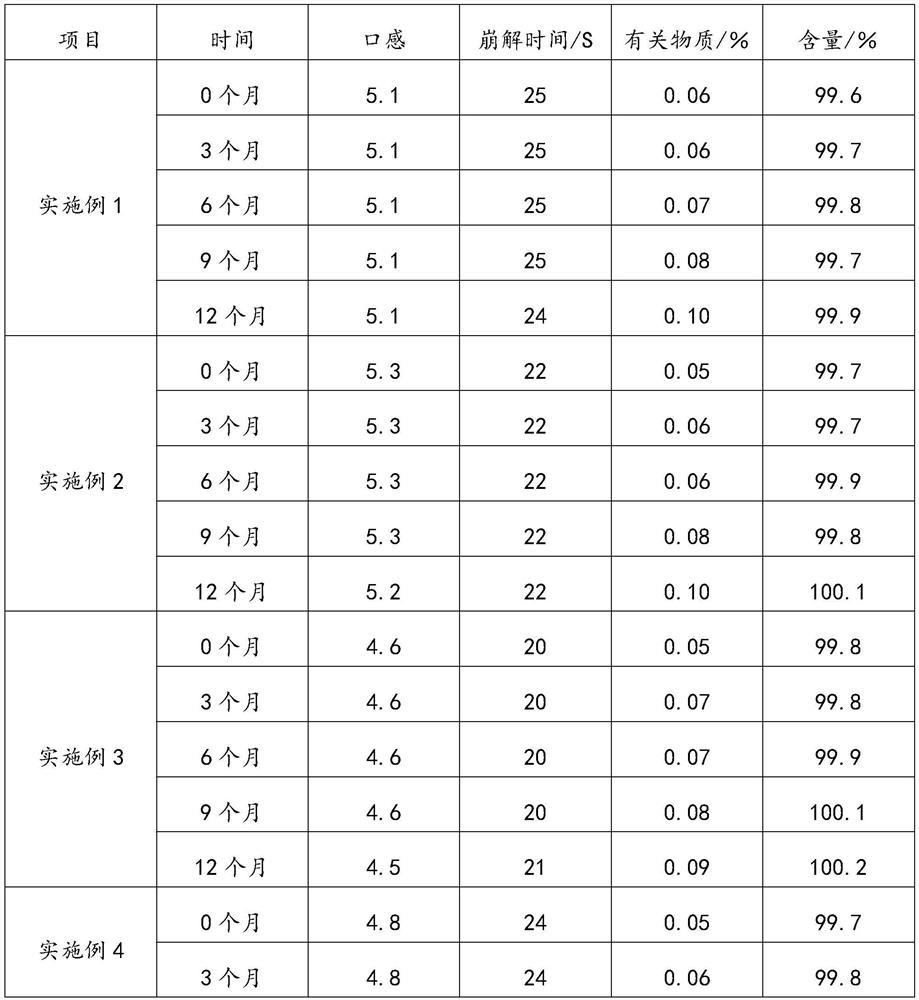
Loratadine oral dispersible film agent and preparation method thereof
ActiveCN112089708AEvenly distributedImprove stabilityOrganic active ingredientsInorganic non-active ingredientsCelluloseBULK ACTIVE INGREDIENT
The invention relates to an loratadine oral dispersible film agent. The loratadine oral dispersible film agent is prepared from the following raw and auxiliary materials in percentage by mass of 25-40% of loratadine, 0.3-1% of polysorbate 80, 1-10% of plasticizer, 0.001-0.02% of glyceride fatty acid, 2-5% of povidone K30, 22-35% of hypromellose-E5, 10-25% of hydroxypropyl cellulose-SL, 1-8% of maltose, 0.5-5% of saccharin sodium, and 1-6% of opacifier; and the particle size X90 of loratadine is less than or equal to 10 mu m. The preparation process disclosed by the invention is simple and controllable, and is suitable for industrial mass production; the prepared oral dispersible film agent has significantly improved tensile strength, disintegration speed, taste and stability, has the advantages of high drug loading capacity, small volume, convenience in carrying, dosage control and administration, and rapid disintegration in the oral cavity without drinking water, realizes rapid release of active ingredients of the drug, has good taste and is easy to accept, and compliance and the safety of children medication are improved.
Owner:JIANMIN PHARMA GRP CO LTD
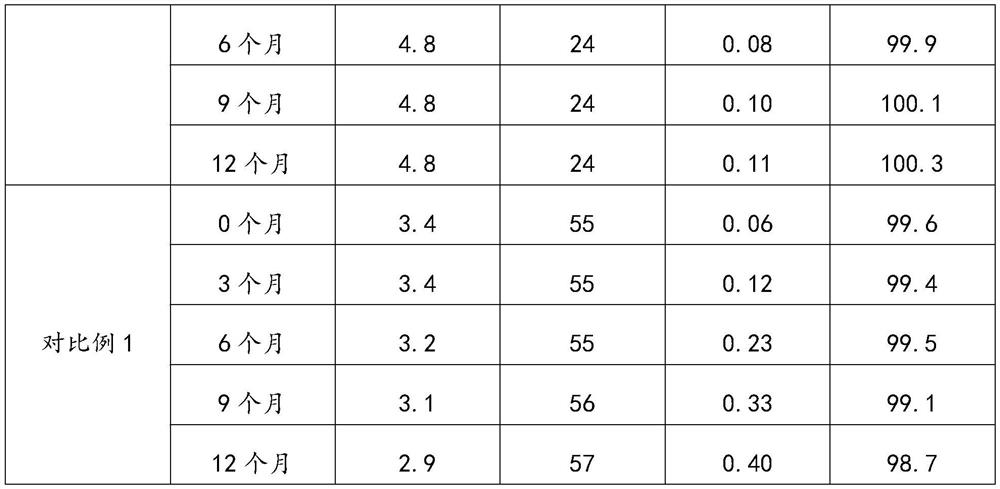
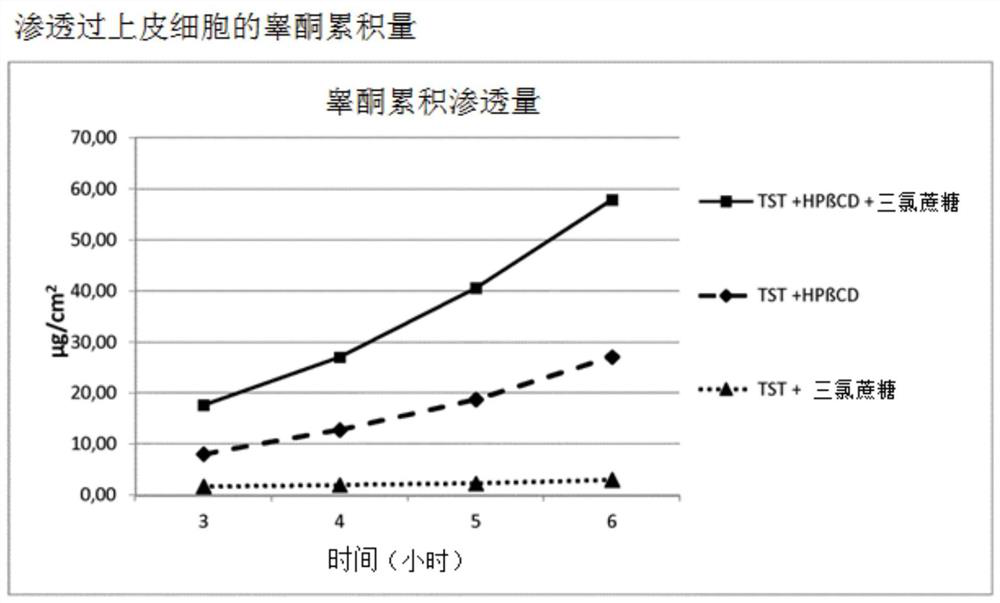
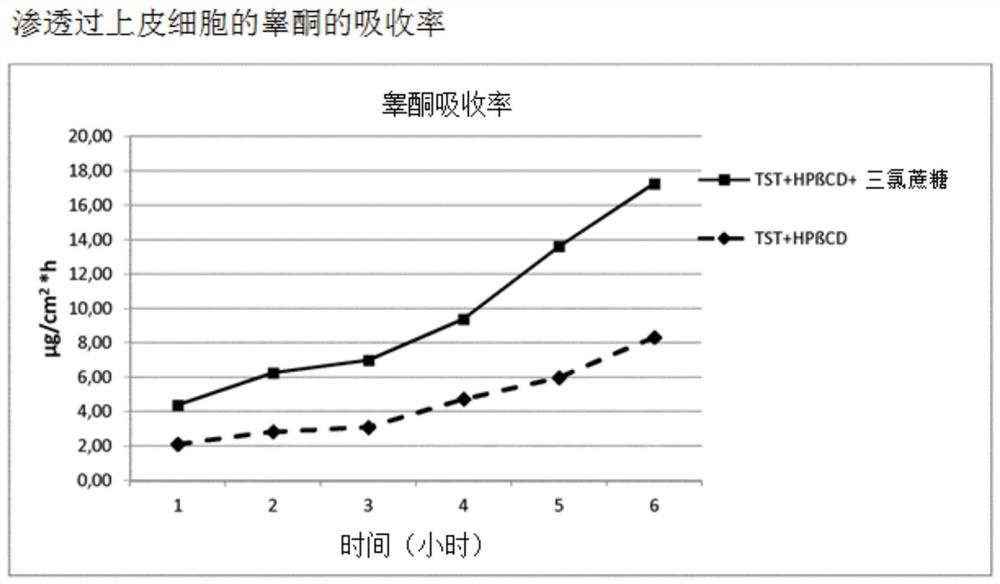
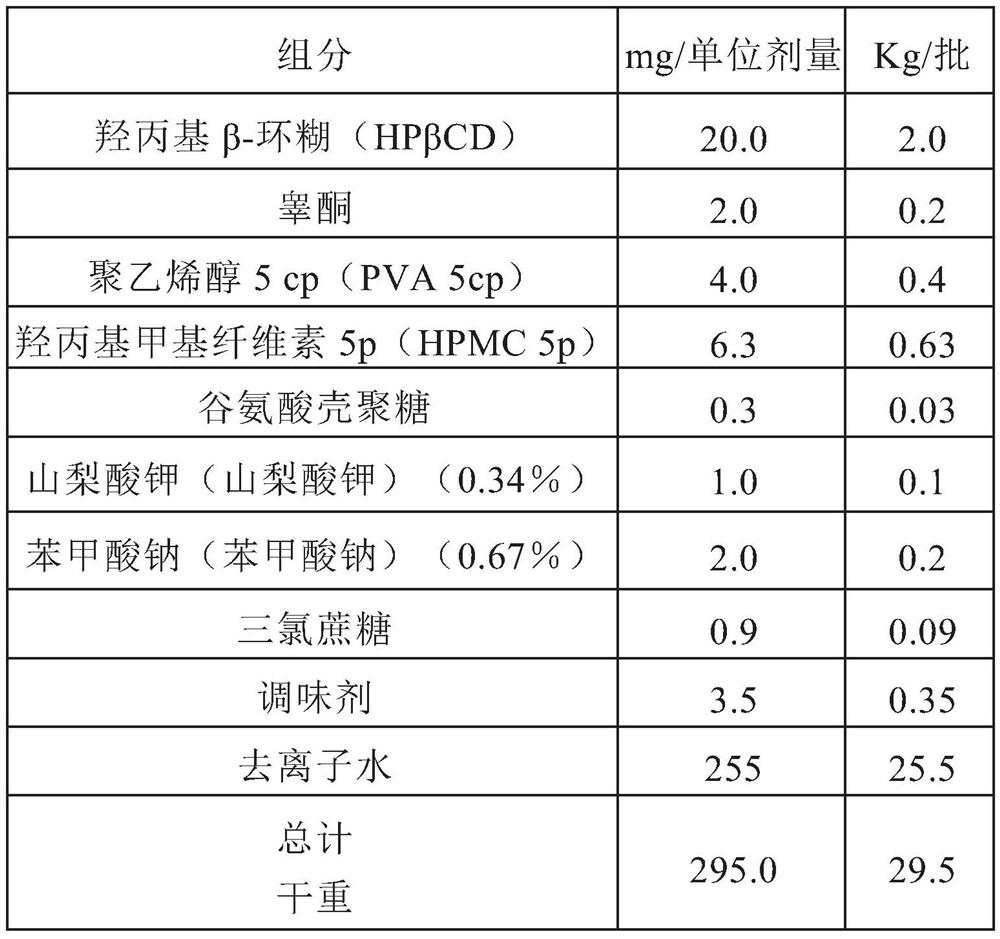
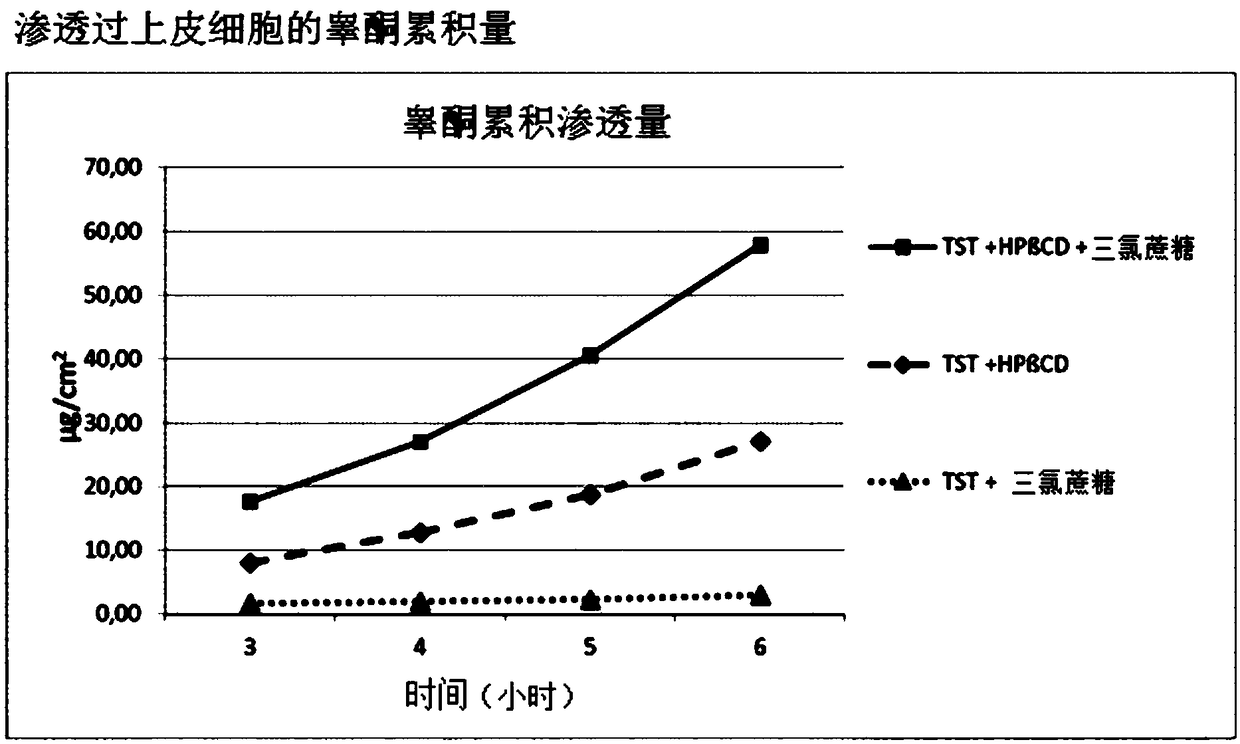
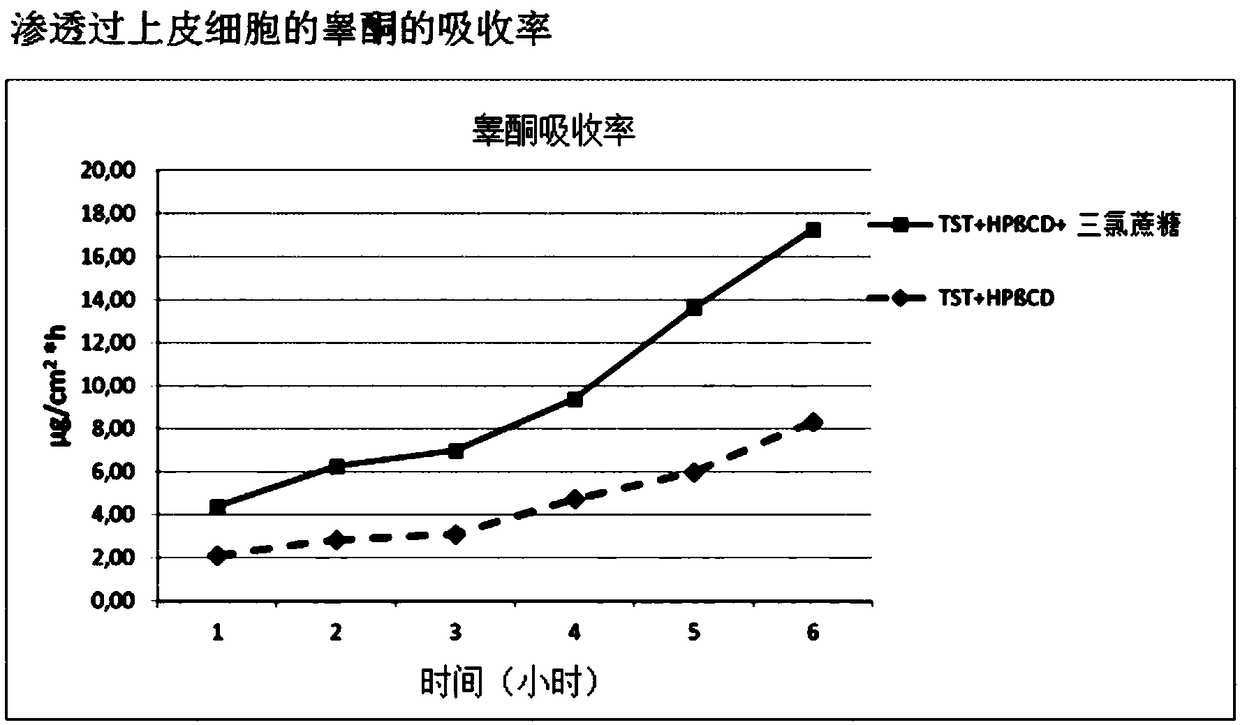
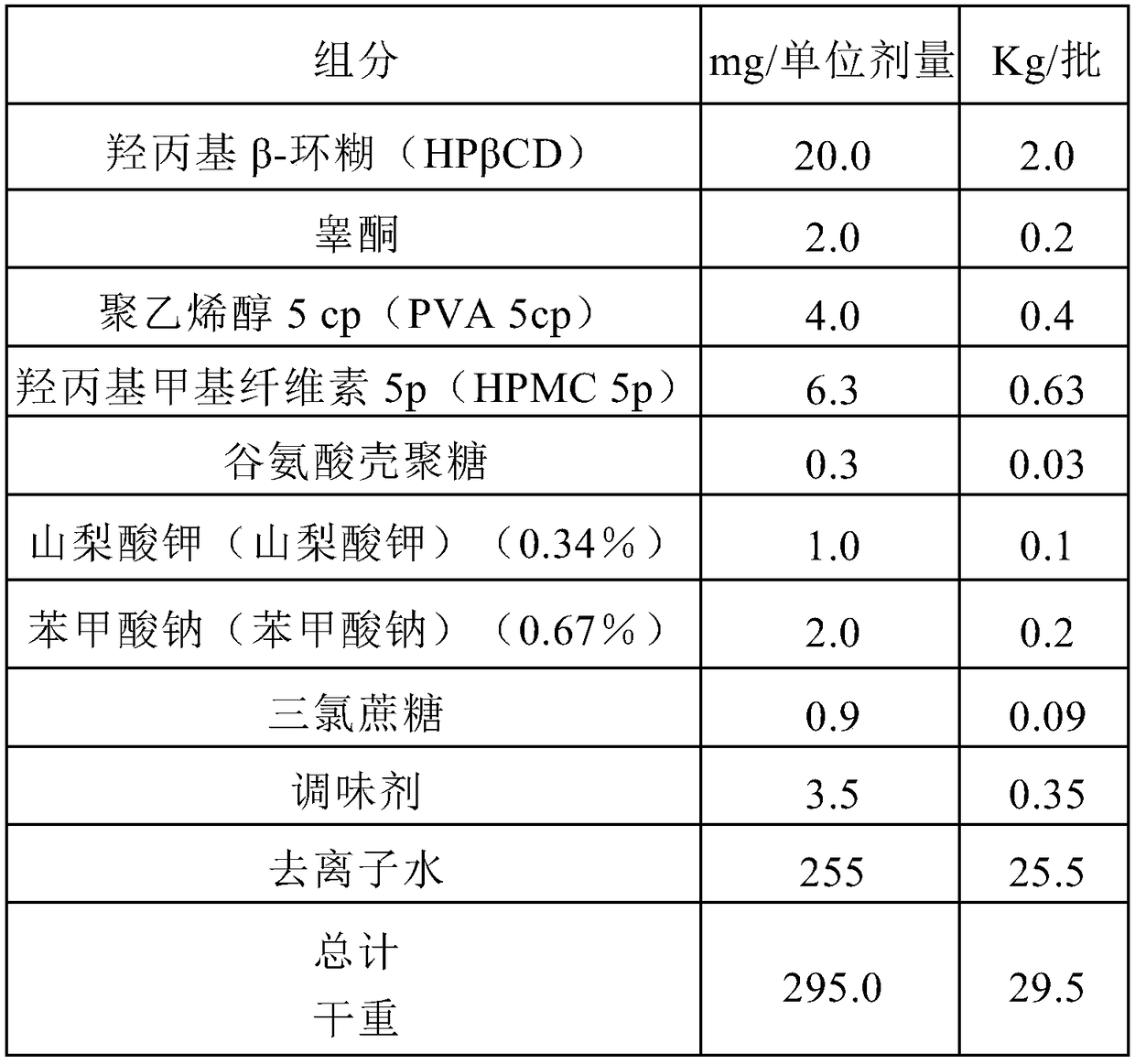
Highly bioavailable oromucosal drug formulations based on cyclodextrin and sucralose
ActiveCN108778342BImprove bioavailabilityOrganic active ingredientsDispersion deliveryOral mucous membraneHigh absorption
Owner:ALTERGON
High bioavailability oromucosal pharmaceutical preparations based on cyclodextrin and sucralose
ActiveCN108778342AImprove bioavailabilityOrganic active ingredientsDispersion deliveryHigh absorptionBULK ACTIVE INGREDIENT
The invention relates to a new composite based on hydroxypropyl-beta-cyclodextrin, sucralose, a pharmaceutically active ingredient (API) complexed in said hydroxypropyl-beta-cyclodextrin, and optionally an aqueous vehicle. The composite is obtainable by a complexation process of the API in hydroxypropyl-beta-cyclodextrin, carried out in the presence of sucralose. The composite ensures a surprisingly high API bioavailability through buccal route of absorption, due to a high absorption rate through the oral membrane. The composite is therefore suitable for the preparation of oromucosal pharmaceutical compositions, e.g. buccal or sublingual tablets, orodispersible film, etc., having high bioavailability.
Owner:ALTERGON
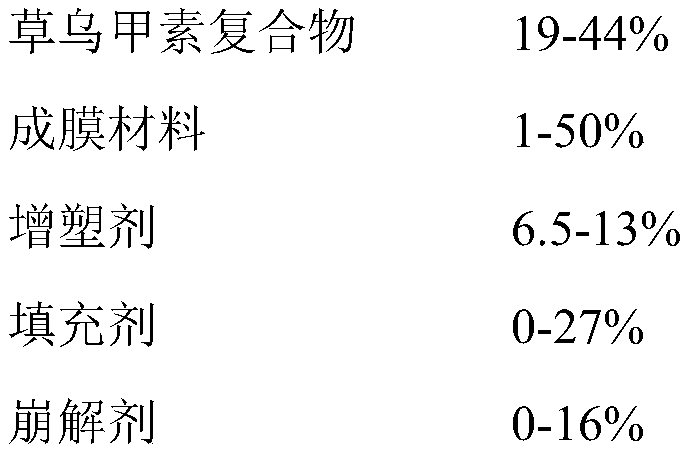
A kind of orodispersible film preparation of aconitin and its preparation process
The invention belongs to the field of pharmaceutical preparations, and discloses an orodispersible film preparation of aconitin and a preparation process thereof. The dispersion film contains aconitin, polyacrylic acid resin No. 2, film-forming matrix, sweetener, saliva stimulating agent and essence. The dispersion film can better cover the numbness and bitterness of the oraconitin, and the preparation method is simple and easy.
Owner:YUNNAN INST OF MATERIA MEDICA
Orodispersible film
InactiveUS20170182105A1Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsEngineeringOrodispersible film
Owner:HEXAL AG
Dextromethorphan hydrobromide oral dispersible film agent and preparation method thereof
ActiveCN103705493BGreat tasteImprove complianceOrganic active ingredientsPharmaceutical non-active ingredientsPolacrilinBiology
Owner:山西皇城相府药业股份有限公司
Orodispersible films having quick dissolution times for therapeutic and food use
ActiveUS11123287B2Prevent hardeningOrganic active ingredientsCosmetic preparationsActive agentBULK ACTIVE INGREDIENT
The present invention concerns an orodispersible self-supporting film free from hydrocolloids comprising: a) a film-forming substance consisting of a maltodextrin in an amount comprised between 40 and 80% by weight; b) a plasticizer in an amount comprised between 15 and 55% by weight; e) a surfactant System in an amount comprised between 0.5 and 6% by weight; d) an active ingredient for food or therapeutic use in an amount between 0.05 and 30% by weight, characterised in that it contains a homopolymer or a copolymer of vinyl acetate in a quantity comprised between 2 and 10% by weight where the percentages are calculated on the total weight of said film.
Owner:PHARMAFILM
Orodispersible film composition comprising enalapril for the treatment of hypertension in a pediatric population
ActiveUS11154585B2Reduce the amount requiredProcess stabilityOrganic active ingredientsDipeptide ingredientsPharmaceutical medicinePediatric population
Owner:PHARMATHEN
Method for preparing oxiracetam oral dispersible film preparation
InactiveCN106822061AUniform appearanceUniform and complete appearanceOrganic active ingredientsNervous disorderPlasticizerHot melt
The invention discloses a method for preparing an oxiracetam oral dispersible film preparation. The method comprises the following steps of sufficiently grinding and uniformly mixing 1 to 15 parts of oxiracetam, 80 to 95 parts of film forming material, 5 to 10 parts of plasticizer, 2 to 5 parts of saliva irritant and 1 to 3 parts of sweetener, sending a mixture to a hot melting zone through a feeding zone of a hot-melt film laminator, hot melting the mixture at 70 to 95 DEG C, continuously outputting the molten mixture through a proportioning zone, pouring the molten mixture into a mold, and cooling the mixture to form the film preparation. According to the method, the film forming material of specific type and use level is selected to be combined with the plasticizer; thus, technical problems that the film preparation is easy to break, is poor in strength and toughness, is slow to disintegrate, is longer in dissolution time and is not beneficial to the absorption of a medicine, and the like, are solved; the made oxiracetam oral dispersible film preparation is enabled to be good in demolding performance; a medicinal film is flexible, is uneasy to break, and is short in the dissolution time.
Owner:CHONGQING RUNZE PHARM CO LTD
Tadalafil orodispersible film and preparation method thereof
ActiveCN107106508BAvoid breakingHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsTadalafilActive agent
The present disclosure relates to a composition for preparing an orodispersible film, which comprises tadalafil or a pharmaceutically acceptable salt thereof, a surfactant, a plasticizer and a sweetener; an orodispersible film; and a preparation method thereof . The orodispersible film of the present disclosure can improve the dissolution rate of tadalafil without a complicated process, and it has excellent stability compared with the previous Cialis because the generation of related compounds is suppressed regardless of the presence or absence of packaging. In addition, when polyethylene glycol 400 is included in a specific content as a plasticizer, excellent film properties can be maintained, including preventing film breakage, securing flexibility, and preventing oil leakage of film formulations.
Owner:SEOUL PHARMA
Structured orodispersible films
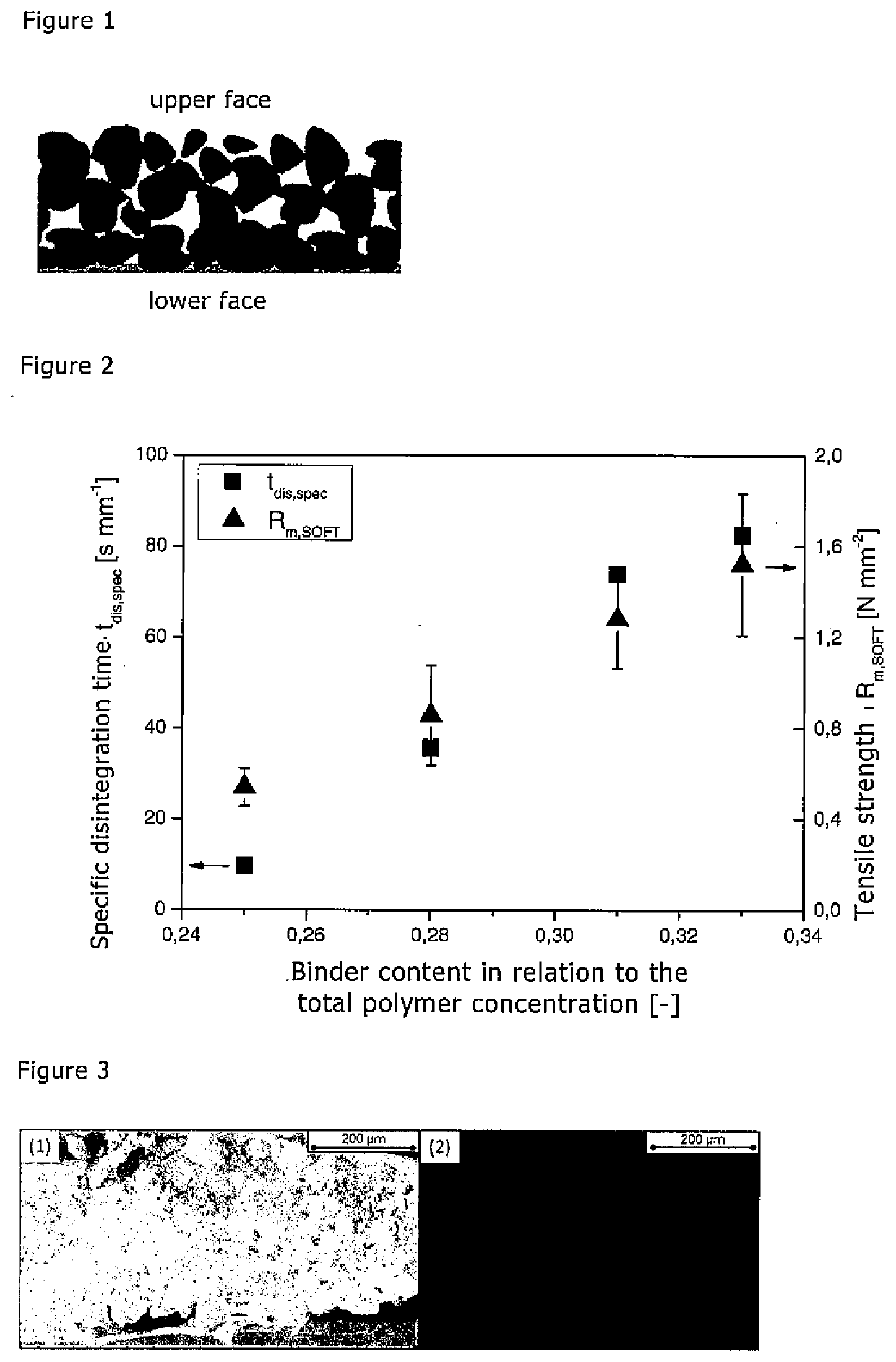
Disclosed is a method for producing a porous orodispersible film, having the following steps: i) forming a suspension of a pharmaceutically acceptable solvent, a pharmaceutically acceptable matrix material, and a pharmaceutically acceptable binder, said solvent being selected such that the pharmaceutically acceptable matrix material substantially does not dissolve in it, whereas the pharmaceutically acceptable binder is dissolved in the solvent, ii) casting the suspension onto a neutral support, thereby forming a wet film, and iii) drying the wet film and obtaining a dry film. The films produced in this manner have a closed surface on the lower face whereas the upper face is porous, thereby allowing the application of a pharmaceutically active ingredient in the form of a suspension or a solution for example. This allows the active ingredient quantity to be adjusted individually to the particular application and produces a film base material which is suitable for the application of different active ingredients.
Owner:LTS LOHMANN THERAPIE-SYST AG
Orodispersible films having quick dissolution times for therapeutic and food use
ActiveUS20150231065A1Prevent hardeningOrganic active ingredientsCosmetic preparationsMedicinePlasticizer
The present invention concerns an orodispersible self-supporting film free from hydrocolloids comprising: a) a film-forming substance consisting of a maltodextrin in an amount comprised between 40 and 80% by weight; b) a plasticizer in an amount comprised between 15 and 55% by weight; e) a surfactant System in an amount comprised between 0.5 and 6% by weight; d) an active ingredient for food or therapeutic use in an amount between 0.05 and 30% by weight, characterised in that it contains a homopolymer or a copolymer of vinyl acetate in a quantity comprised between 2 and 10% by weight where the percentages are calculated on the total weight of said film
Owner:PHARMAFILM
Warfarin sodium oral cavity dispersion film agent and preparation method thereof
InactiveCN108478543AEasy doseUniform thicknessPharmaceutical non-active ingredientsBlood disorderWarfarin SodiumPlasticizer
The invention discloses a warfarin sodium oral cavity dispersion film agent easy for dose deviding, and a preparation method thereof, which belong to the technical field of medicines. The designed warfarin sodium oral cavity dispersion film agent is prepared from warfarin sodium, a film-forming material and a plasticizer. According to the preparation method provided by the invention, the film agent is prepared through a flow casting method, is smooth and clean in surface, and can be quickly dissolved to release medicine when encountering water, so that the warfarin sodium is favorably orally absorbed. In order to achieve the aim of easiness in dose deviding, scale identification is carried out on a warfarin sodium oral cavity dispersion film, and the dosage can be flexibly adjusted according to the length of the film, so that the use is convenient. Through preparing the warfarin sodium oral cavity dispersion film agent, the defects that dose deviding on a traditional warfarin sodium tablet is not easy and is inaccurate in clinic treatment are overcome, and the warfarin sodium oral cavity dispersion film agent is an oral anticoagulant more suitable for clinic personalized medication.
Owner:CHINA PHARM UNIV
Orodispersible film
InactiveUS10335443B2Pharmaceutical delivery mechanismPharmaceutical non-active ingredientsEngineeringOral cavity
Owner:HEXAL AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com