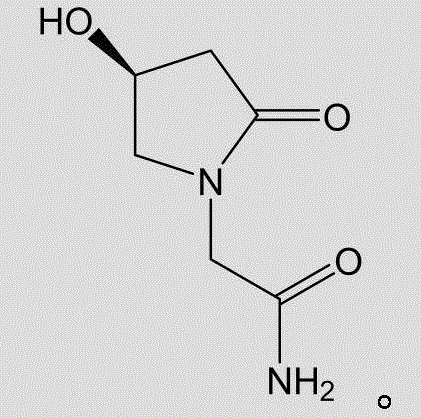
Method for preparing levorotatory oxiracetam oral dispersible film preparation
A technology of dispersing film and oral cavity, which is applied in the field of preparing levo-oxiracetam oral dispersible film, can solve the problems of harm to patients, low drug loading, large solid particle agglomeration, etc., and achieve the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
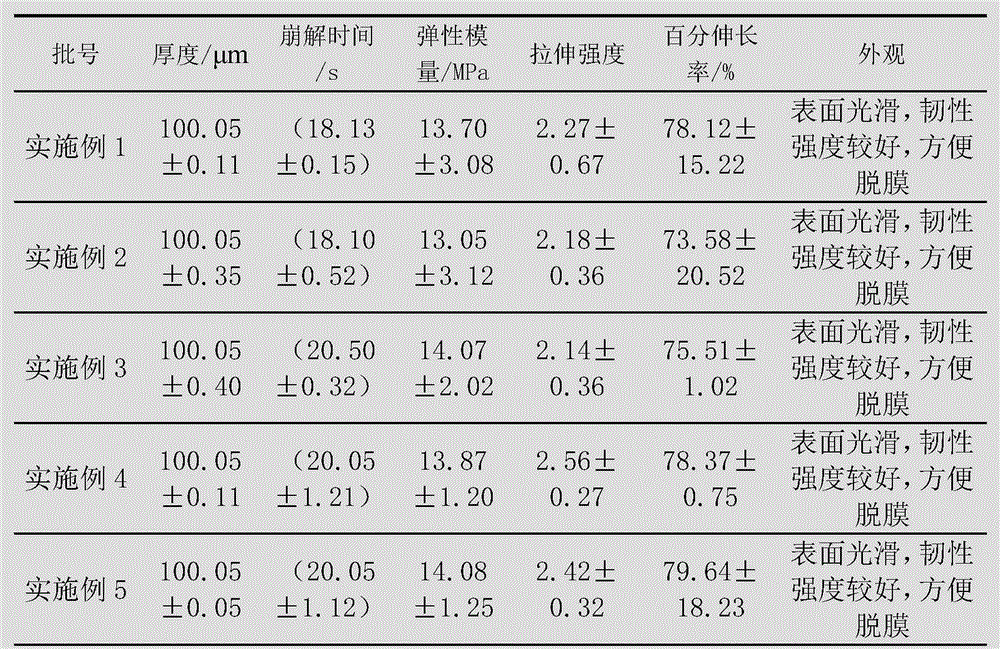
Embodiment 1
[0078] The preparation of levoxiracetam orodispersible film, adopts following steps:
[0079] 1) Dissolve 60g of film-forming material (a combination of acrylate and hydroxypropylmethylcellulose, where the dosage of hydroxypropylmethylcellulose is 30g) with 80mL of absolute ethanol, and remove the air bubbles to obtain a uniform viscous liquid;
[0080] 2) Disperse 15g of glycerin, 20g of microcrystalline cellulose, 4g of malic acid and 2g of sorbitol with 50mL of absolute ethanol to form a dispersion;
[0081] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 5g of levoxiracetam to disperse evenly, and then let it stand to remove air bubbles;
[0082] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 50cm / min, then dry at 65-68°C, and peel off.
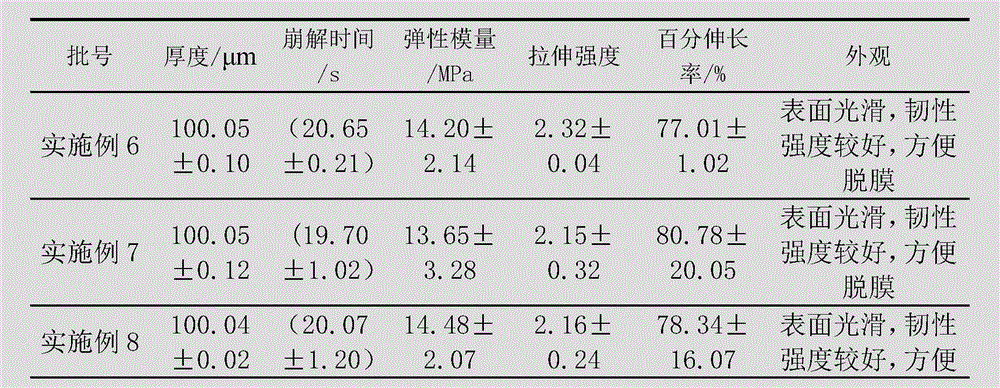
Embodiment 2
[0084] The preparation of levoxiracetam orodispersible film, adopts following steps:
[0085] 1) Dissolve 40g of film-forming material (a combination of acrylate and hydroxypropylmethylcellulose, wherein the dosage of hydroxypropylmethylcellulose is 10g) with 50mL of absolute ethanol, and remove the air bubbles to obtain a uniform viscous liquid;
[0086] 2) Disperse 20g of propylene glycol, 20g of low-substituted hydroxypropyl cellulose, 2g of citric acid and 1g of xylitol with 60mL of absolute ethanol to form a dispersion;
[0087] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 18g of levoxiracetam to disperse evenly, then let it stand to remove air bubbles;
[0088] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 80cm / min, then dry at 70-72°C, and peel off.
Embodiment 3
[0090] The preparation of levoxiracetam orodispersible film, adopts following steps:
[0091] 1) Dissolve 50g of film-forming material (a combination of acrylate and hydroxypropyl methylcellulose, where the dosage of hydroxypropylmethylcellulose is 20g) with 50mL of absolute ethanol, and remove air bubbles to obtain a uniform viscous liquid;
[0092] 2) Disperse 15g of triethyl citrate, 20g of microcrystalline cellulose, 3g of ascorbic acid and 1g of sorbitol with 40mL of absolute ethanol to form a dispersion;
[0093] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 15g of levoxiracetam to disperse evenly, then let it stand to remove air bubbles;
[0094] 4) Coat the viscous liquid after removing air bubbles with a drug film coating dryer at a coating speed of 70cm / min, then dry at 80-85°C, and peel off.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com