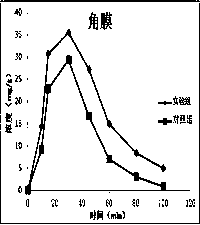
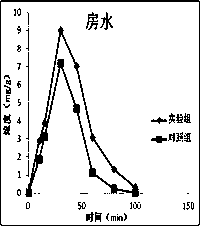
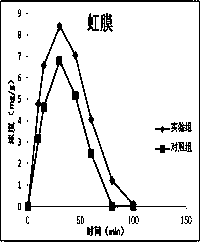
Atropine eye drop having eye posterior targeting function
A technology of atropine and eye drops, applied in the field of medicine, can solve the problems of high-priced ophthalmology patients, discomfort and infection, retinal detachment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Preparation process: put the prescribed amount of γ-cyclodextrin in a vial, add distilled water to dissolve, add NaCl and polyvinyl alcohol under stirring condition, stir continuously to dissolve, and autoclave at 121°C for 15 minutes to obtain Gamma-cyclodextrin sterile solution, then dissolve atropine in dichloromethane, filter and sterilize with a 0.22 μm filter membrane under sterile conditions, obtain sterilized atropine solid under sterile conditions, The atropine is added into the gamma cyclodextrin solution for stirring, and after continuous stirring to form a suspension, the suspension is freeze-dried. Prepare a buffer solution of potassium dihydrogen phosphate and disodium hydrogen phosphate, autoclave at 121°C for 15 min, and perform aseptic filling. Disperse with a buffer solution before use to obtain atropine eye drops.
Embodiment 2
[0025]
[0026] Preparation process: Take the prescribed amount of γ-cyclodextrin / hydroxypropyl γ-cyclodextrin (80:20) in a vial, add distilled water to dissolve, add NaCl and polyvinyl alcohol under stirring conditions, and continuously stir to dissolve it. Autoclave at 121°C for 15 minutes to obtain a sterile solution containing γ-cyclodextrin, then dissolve atropine in dichloromethane, filter and sterilize with a 0.22 μm filter membrane under sterile conditions, and obtain For the sterilized atropine solid, the atropine is added into the gamma cyclodextrin solution under aseptic conditions for stirring, and after continuous stirring to form a suspension, the suspension is freeze-dried. Prepare a buffer solution of potassium dihydrogen phosphate and disodium hydrogen phosphate, autoclave at 121°C for 15 min, and perform aseptic filling. Disperse with a buffer solution before use to obtain atropine eye drops.
Embodiment 3
[0028]
[0029] Preparation process: Put the prescribed amount of γ-cyclodextrin / hydroxypropyl γ-cyclodextrin (80:20) in a vial, add distilled water to dissolve, add NaCl and polyvinyl alcohol under stirring conditions, and continuously stir to dissolve it , autoclave at 121°C for 15 minutes to obtain a sterile solution containing γ-cyclodextrin, then dissolve atropine in dichloromethane, filter and sterilize with a 0.22 μm filter membrane under sterile conditions, and sterilize under sterile conditions After obtaining the sterilized atropine solid, the atropine is added into the gamma cyclodextrin solution under aseptic conditions for stirring, and after continuous stirring to form a suspension, the suspension is freeze-dried. Prepare a buffer solution of potassium dihydrogen phosphate and disodium hydrogen phosphate, autoclave at 121°C for 15 min, and perform aseptic filling. Disperse with a buffer solution before use to obtain atropine eye drops.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com