Use of human urinary kininogenase in preparation of medicine for treating migraine and its composition
A technology of kininogenase and composition, which is applied in the field of medicine, can solve problems such as adverse reactions, and achieve the effects of reducing the incidence of adverse reactions, good stability, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
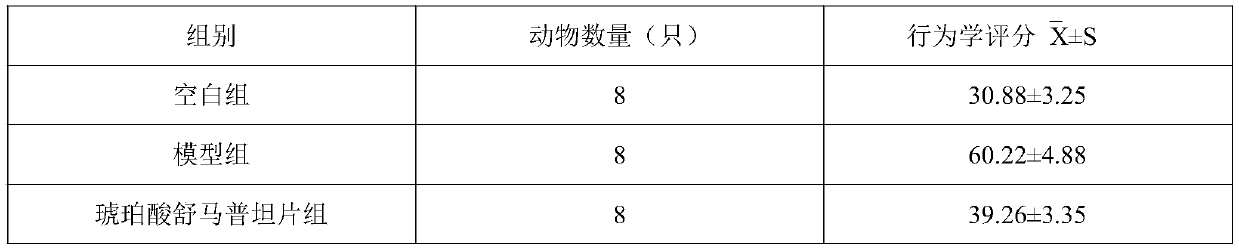
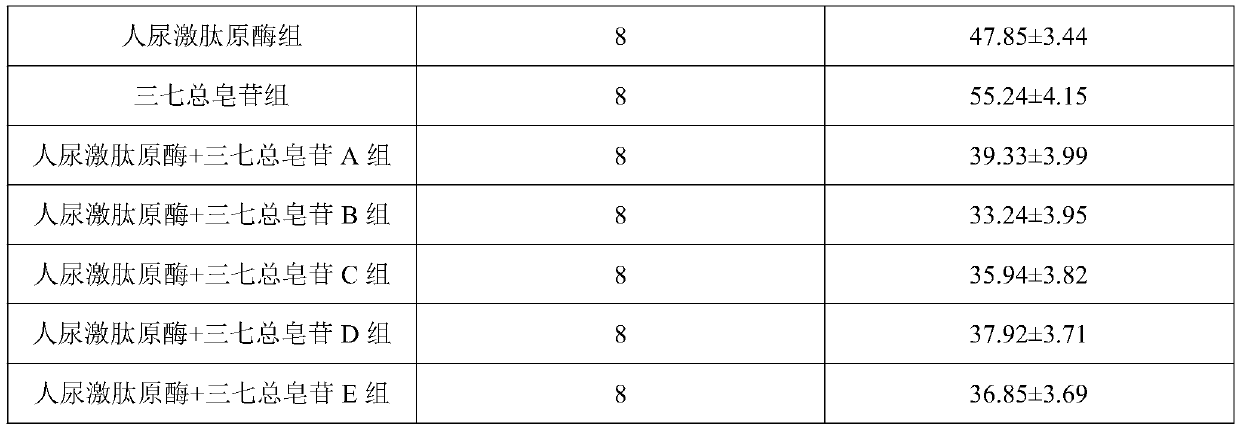
[0019] Embodiment 1, human urinary kallikrein injection of the present invention
[0020] Take filter-sterilized human urinary kininogenase 150PNA units, add an appropriate amount of water for injection to dissolve, adjust the pH value to neutral, add water for injection to 1000mL, add sodium chloride to adjust isotonicity, filter aseptically, and pack 1000 pieces In ampoules, you can.
Embodiment 2
[0021] Embodiment 2, the present invention treats the injection of migraine
[0022] Take filter-sterilized human urinary kininogenase 72PNA unit, add an appropriate amount of water for injection to dissolve, then add 3g of Panax notoginseng saponins, dissolve, adjust the pH to neutral, add water for injection to 1000mL, add sodium chloride to adjust isotonicity , sterile filtered, and packed in 1000 ampoules.
Embodiment 3
[0023] Embodiment 3, the present invention treats the injection of migraine
[0024] Take filter-sterilized human urinary kininogenase 150PNA units, add an appropriate amount of water for injection to dissolve, then add 4.5g of Panax notoginseng saponins, dissolve, adjust the pH to neutral, add water for injection to 1000mL, add sodium chloride to adjust, etc. Infiltration, sterile filtration, and packing into 1000 ampoules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com