Tripterine derivative and application thereof
A halogen and compound technology, applied in the field of medicine, can solve problems such as complex etiology of cancer, toxic and side effects of anticancer drugs, and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
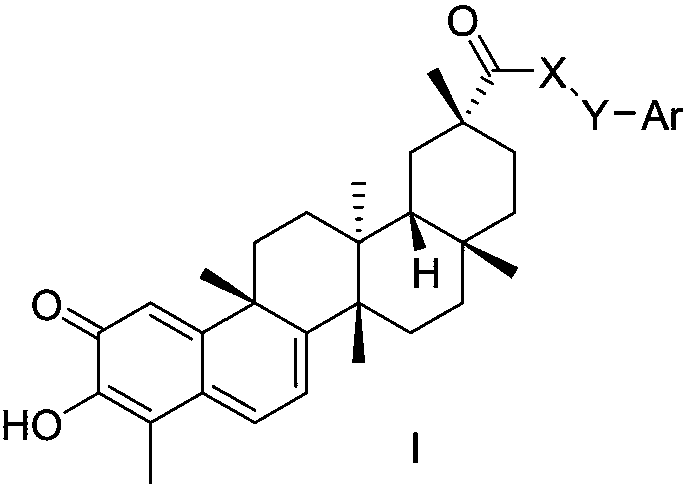
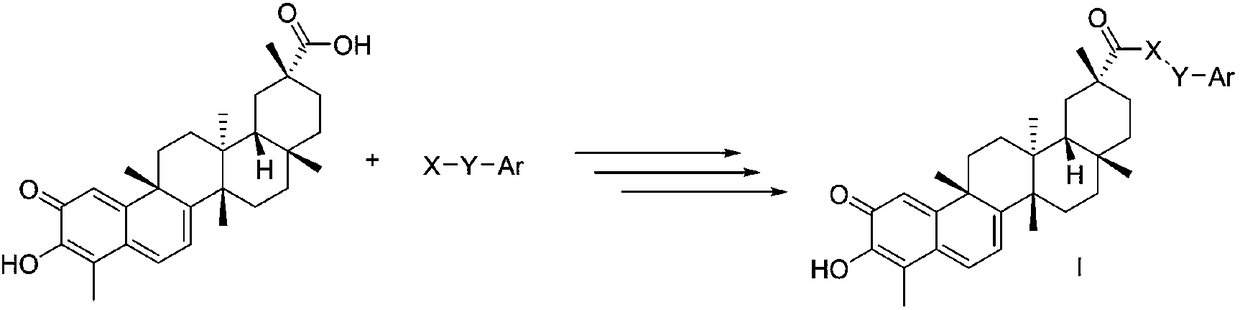
[0055] X is Y is n=2; Ar is different R 1 When substituted phenyl, the preparation of compound I-1~I-12
[0056] Step A:
[0057]
[0058] Weigh 5mmol of substituted trans-cinnamic acid derivative 1, slowly add 10ml of SOCl 2 , stirred at room temperature for 1h, if the reactant is not dissolved, heat to 40°C and stir for 0.5h, evaporate the solvent to obtain substituted cinnamic acid chloride; then add a small amount of anhydrous dichloromethane to dissolve, and transfer the obtained solution to a constant pressure dropping funnel , weighed 10mmol piperazine and dissolved it in 20ml glacial acetic acid, slowly added the solution in the constant pressure dropping funnel dropwise to piperazine, and stirred at room temperature for 2h. The reaction solution was adjusted to pH 7-9 with 40% NaOH, extracted 3 times with dichloromethane, the organic phases were combined, washed 3 times with saturated NaCl, dried over anhydrous sodium sulfate, and concentrated under reduced p...
Embodiment 2
[0076] X is Y is n=2; Ar is different R 1 When substituted phenyl, the preparation of compound I-13~I-24
[0077] Step A:
[0078]
[0079] Weigh 5mmol of substituted trans-cinnamic acid derivative 1, slowly add 10ml of SOCl 2 , stir at room temperature for 1 hour, if the reactant is not dissolved, heat to 40°C and stir for 0.5 hour, evaporate the solvent to dryness to obtain substituted cinnamic acid chloride, then add 10ml of anhydrous dichloromethane to dissolve, add dropwise 3ml of methanol, and stir at room temperature for 10min. Concentrate under reduced pressure to obtain compound 4; add 20ml of ethylenediamine to the obtained compound 4, reflux for 2h, let cool to room temperature, pour the reaction solution into water, extract 3 times with ethyl acetate, combine the organic phases, and use saturated Washed three times with NaCl, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a yellow oil with a yield of 52-73%.
[0080...
Embodiment 3
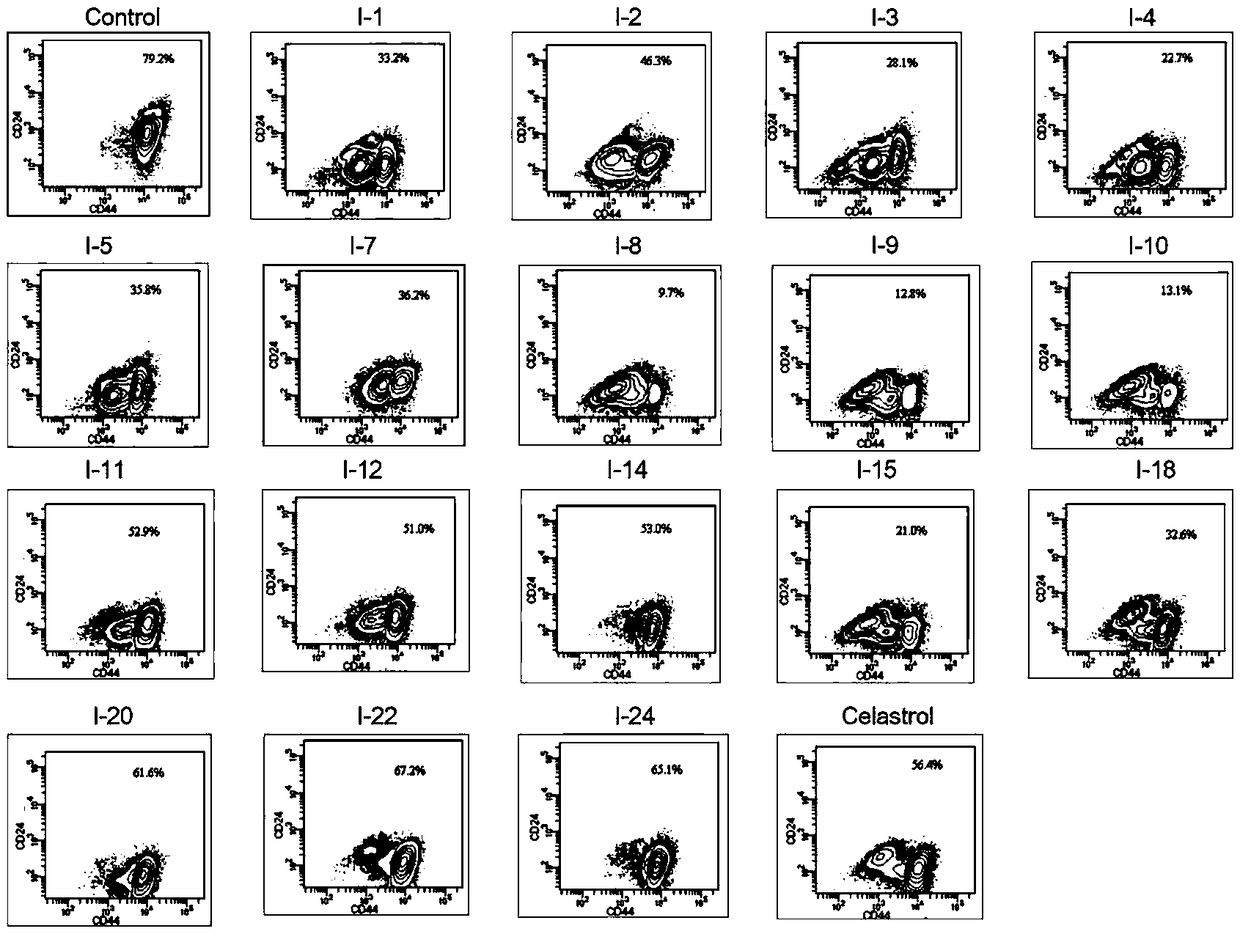
[0094] Inhibitory effect of tripterine derivatives of the present invention on tumor cell growth
[0095] MTT assay was used to detect the growth inhibitory effect of ovarian cancer cells SKOV-3 and OVCAR3. Will 2×10 3 Cells were inoculated in a 96-well plate, and allowed to adhere to the wall and grow for 48 hours before adding the test drugs at various concentrations. After culturing, add 50 μL of MTT solution (2 mg / mL) to each well, continue to incubate for 4 hours, shake the plate to remove the culture solution, add DMSO, and measure its absorbance at 570 nm. Obtain the cell growth inhibition rate caused by the test drug by the absorbance ratio (%) of the drug-dosed group and the blank control group, and calculate the IG 50 , the results are shown in Table 1.
[0096] Table 1. Growth Inhibitory Activity of Compounds on Tumor Cells
[0097]
[0098]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com