Terpenoid phenolic compound and application of terpenoid phenolic compound and cis-platinum in anti-tumor medicines
A technology of anti-tumor drugs and compounds, applied in anti-tumor drugs, pharmaceutical formulations, organic chemistry, etc., can solve problems such as multi-drug resistance of tumors that are difficult to achieve therapeutic effects, achieve good anti-tumor cell proliferation activity, and inhibit tumor cells Proliferation effect is obvious and the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of a compound, comprising the steps of:
[0033] Take a 10ml reaction bottle, add the substrates THC (330mg, 1.05mmol), p-toluenesulfonic acid (400mg, 2.10mmol), toluene (5ml) and put it in a 60°C oil bath, react for 24h, and the reaction is completed by HPLC detection. Cool to room temperature, add saturated sodium bicarbonate to the reaction system to quench the reaction, extract with ether, combine the organic phases, wash with saturated brine and dry over anhydrous sodium sulfate, concentrate under reduced pressure, put on the column (ordinary silica gel: 300-400 mesh, self-filled Purified by chromatography (n-hexane: acetone = 25:1) to obtain the target compound (210 mg, yield 64%).
[0034] Structural analysis of the target compound: Finally, after mass spectrometry, nuclear magnetic resonance and other data, the structure of the compound was comprehensively identified as shown in formula Ⅰ:
[0035]
[0036] The target compound data infor...
Embodiment 2
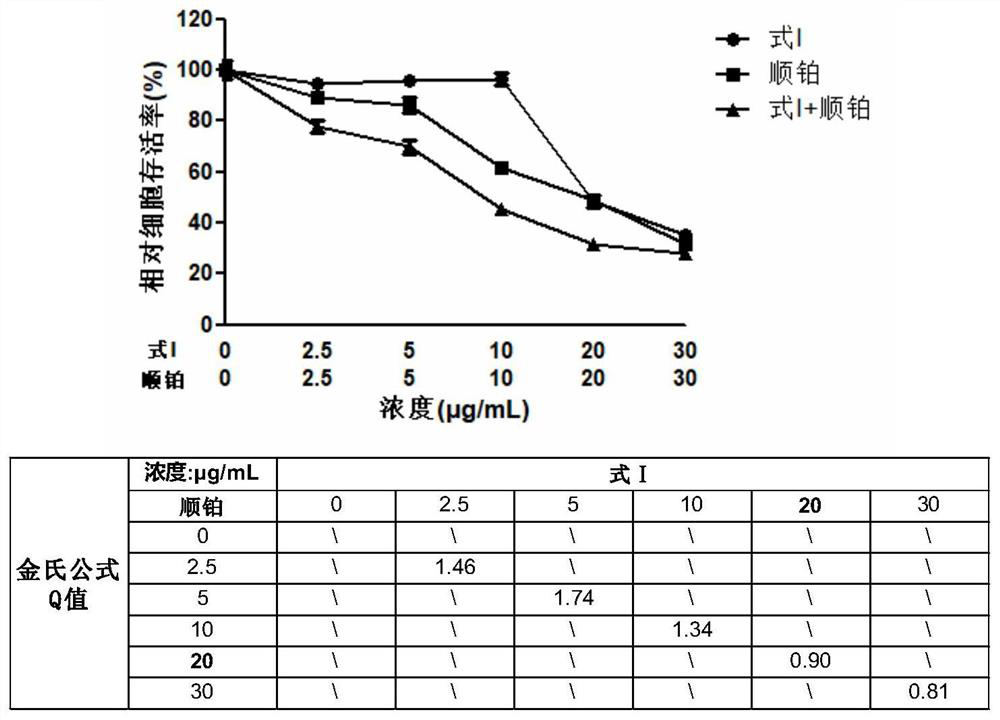
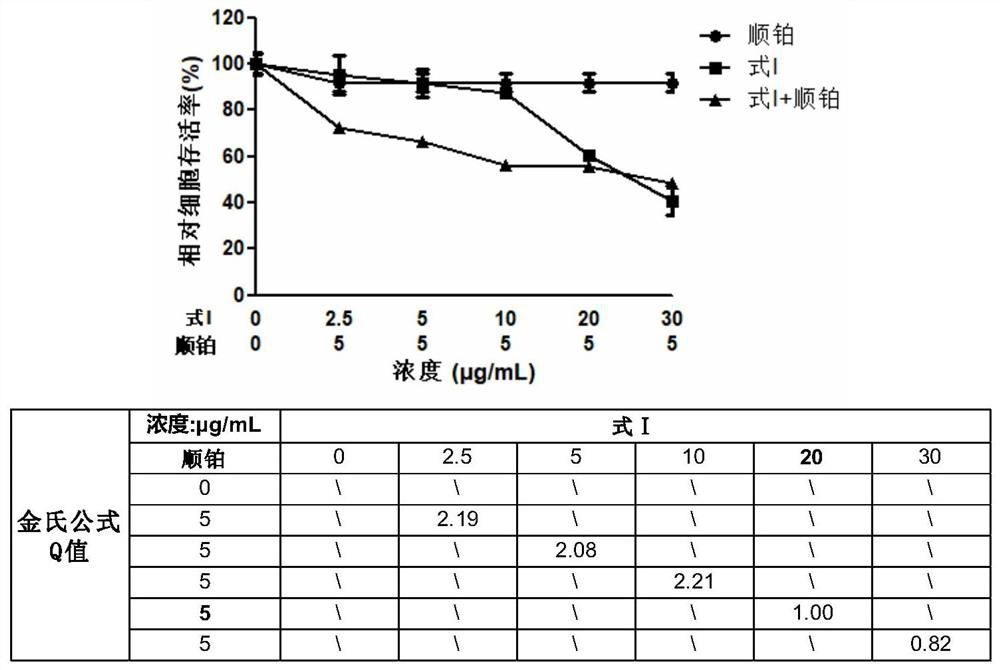
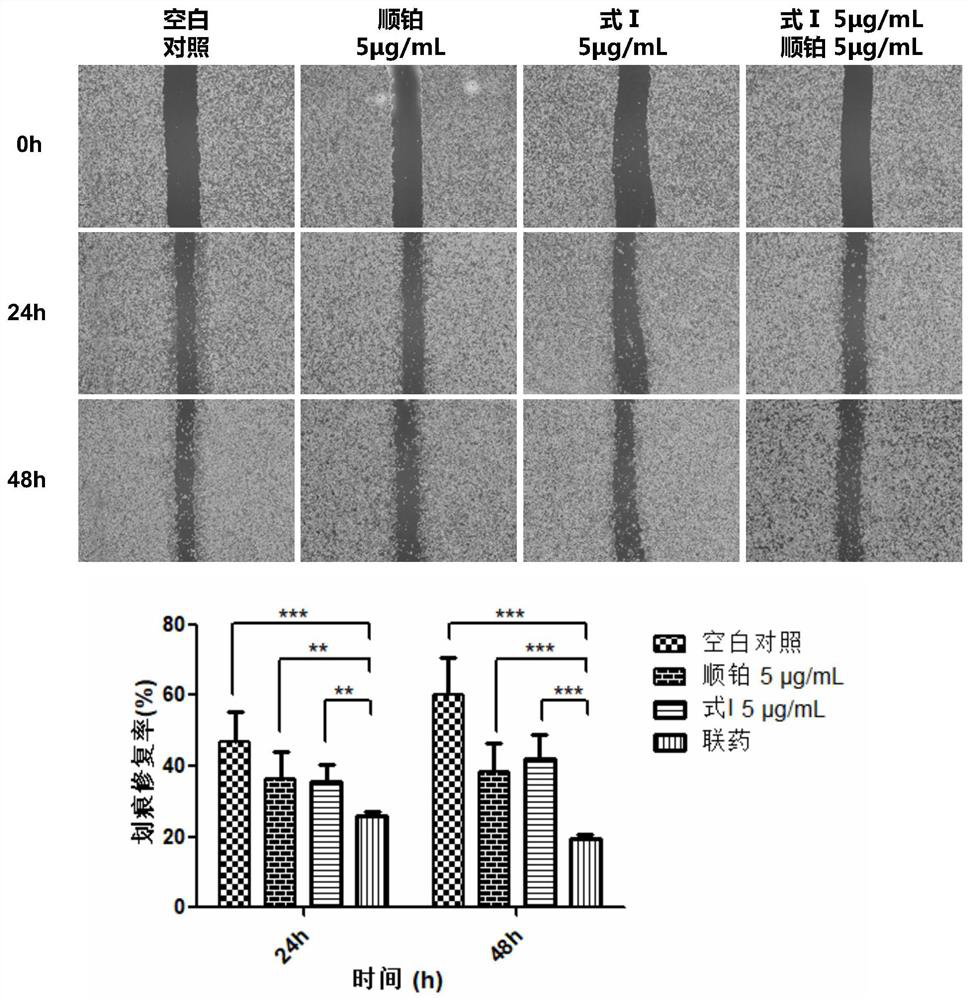
[0040] Combination drug anti-tumor cell proliferation activity assay method:
[0041] (1) CKK8 colorimetric method to detect the effect of combined drug on the survival performance of tumor cells
[0042] Take human liver cancer cells (HepG2) in the logarithmic growth phase, and prepare a cell suspension with a suitable concentration in DMEM culture medium. The cells were seeded in 96-well plates and cultured in a 37°C incubator until the cells adhered to the wall. Using DMSO as a solvent, a solution of the compound shown in Formula I and a cisplatin compound solution with a concentration of 20 mg / mL were prepared respectively, and diluted with culture medium to the required working concentration during the experiment. After discarding the culture medium in the 96-well plate, 100 μL of cisplatin solutions with working concentrations of 2.5 μg / mL, 5 μg / mL, 10 μg / mL, 20 μg / mL and 30 μg / mL were added to the single-drug group respectively; the other single-drug group was added wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com