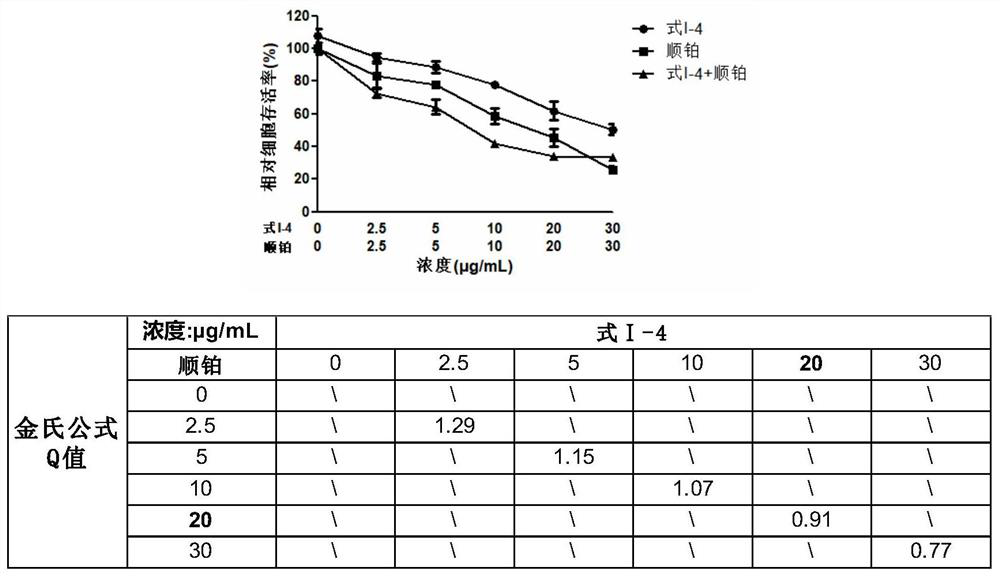
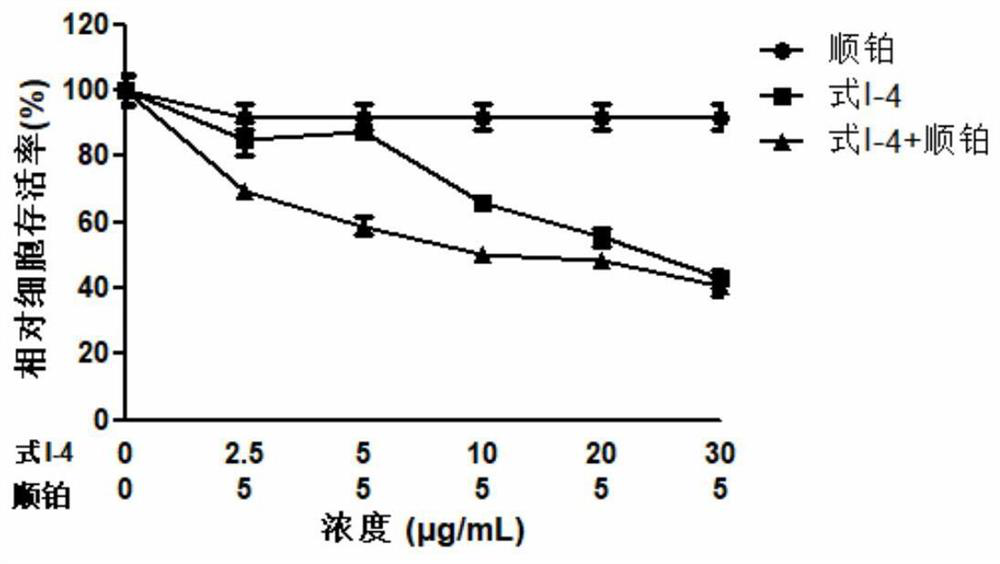
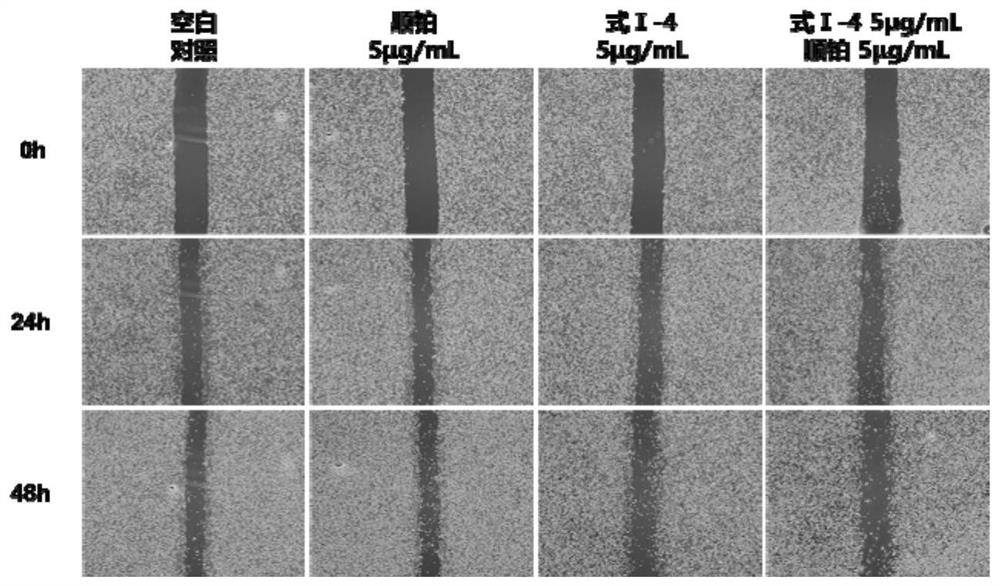
2,6-substituted-4-oxyterpene phenol pyridine compound as well as preparation method and application thereof
A technology of oxyterpene phenol pyridine and compound, which is applied in the field of pharmaceutical compounds, can solve problems such as limiting the clinical application of cisplatin, and achieve good anti-tumor cell proliferation activity, high purity, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Take a 10mL reaction bottle, add CBD (100mg, 0.32mmol), chloride (108.6mg, 0.32mmol), solvent DMF (1mL) in sequence, mix well, remove oxygen, add Cs 2 CO 3 (104 mg, 0.32 mmol), placed in a 90°C oil bath, reacted for 24 hours, TLC detected the completion of the reaction, cooled to room temperature, placed in an ice-water bath, added 1N HCl to the system to adjust the pH to 3-5, and extracted with ether. The organic phases were combined, washed once with saturated sodium bicarbonate, washed once with saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and separated by column chromatography (200-300 mesh silica gel; n-hexane: ethyl acetate = 2:1) to obtain the crude product , using (300-400 mesh silica gel; n-hexane: acetone = 2:1) to obtain the target compound (17.6 mg, yield 11%). 1 H NMR (400MHz, CDCl 3 )δ8.59(d, J=5.6Hz, 2H), 7.63(s, 2H), 6.93(d, J=3.3Hz, 2H), 6.73(s, 2H), 4.89(s, 1H), 4.49( s,1H),4.47(s,1H),4.41(t,...
Embodiment 2
[0058]
[0059] Referring to Example 1 for the synthetic steps of compound I-2, the target compound I-2 (17.8 mg, yield 11.6%) was obtained. 1 H NMR (400MHz, CDCl 3 )δ8.57(d, J=5.6Hz, 1H), 7.63(d, J=2.3Hz, 1H), 6.92(dd, J=5.6, 2.5Hz, 1H), 6.59(s, 1H), 6.33( s,1H),6.04(brs,1H),5.46(brs,1H),5.38–5.24(m,1H),4.52(s,1H),4.40(s,1H),3.70–3.55(m,1H) ,2.57–2.41(m,3H),2.30–2.14(m,1H),2.12–1.99(m,1H),1.84–1.63(m,5H),1.57–1.51(m,2H),1.48(s, 3H), 1.41(d, J=6.3Hz, 6H), 1.34–1.26(m, 4H), 0.86(t, J=6.9Hz, 3H). MS(ESI) m / z: 478[M+H] + .
Embodiment 3
[0061]
[0062] Referring to Example 1 for the synthetic steps of compound I-3, the target compound I-3 (21 mg, yield 13.4%) was obtained. 1 H NMR (400MHz, CDCl 3 )δ8.56(d, J=5.6Hz, 1H), 7.63(d, J=2.3Hz, 1H), 6.93(dd, J=5.5, 2.4Hz, 1H), 6.60(s, 1H), 6.33( s,1H),6.05(brs,1H),5.46(brs,1H),4.51(s,1H),4.41(s,1H),4.39(t,J=6.8Hz,2H),3.70–3.55(m ,1H),2.55–2.43(m,3H),2.29–2.14(m,1H),2.12–1.98(m,1H),1.84–1.63(m,7H),1.59–1.51(m,2H),1.51 –1.37(m,5H),1.34–1.21(m,4H),0.96(t,J=7.4Hz,3H), 0.87(t,J=6.9Hz,3H).MS(ESI)m / z:492 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com