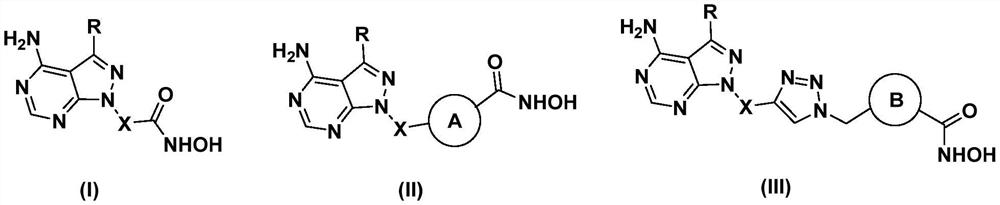
A class of mtor/hdac dual inhibitors and their applications
A dual inhibitor and dosage form technology, applied in the field of mTOR/HDAC dual inhibitor composition, can solve problems such as inability to terminate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
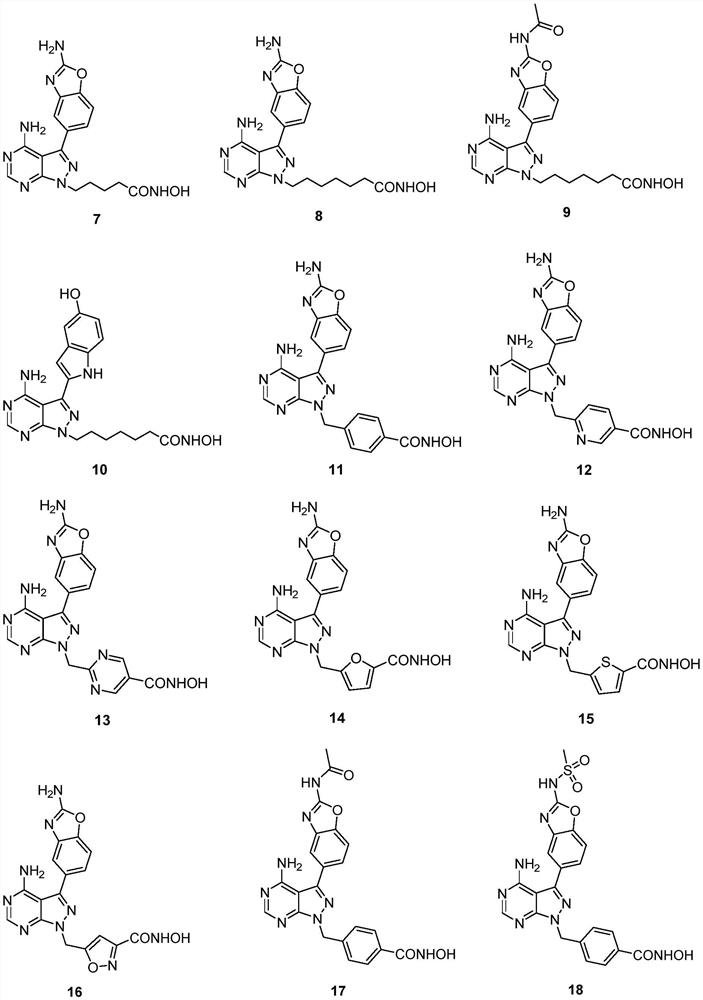
Image
Examples
Embodiment 1
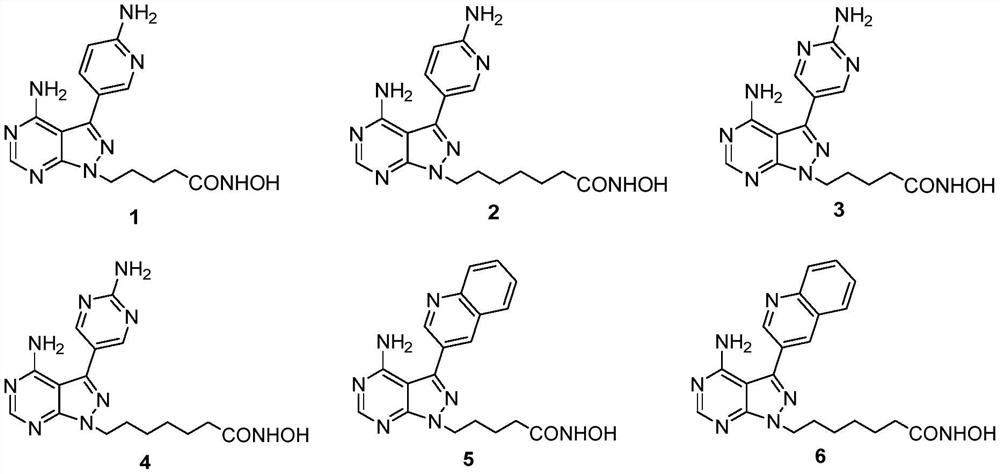
[0059] Synthesis of 5-(4-amino-3-(2-aminopyrimidine-5-)-1H-pyrazolo[3,4-d]pyrimidine-1)-N-hydroxypentanamide (compound 3).
[0060] (1) Synthesis of ethyl 5-(4-amino-3-iodo-1H-pyrazolo[3,4-d]pyrimidine-1-)valerate (intermediate 24):
[0061] Add 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (1.5g), ethyl 5-bromovalerate (1.3g), anhydrous potassium carbonate (1.1 g) and anhydrous DMF (20 mL), stirred at 60°C for 12 hours. Add an appropriate amount of water to the system, let it stand, and filter with suction to obtain a crude product, which is 1.7 g of an off-white solid through silica gel column chromatography.
[0062] (2) Synthesis of ethyl 5-(4-amino-3-(2-aminopyrimidine-5-)-1H-pyrazolo[3,4-d]pyrimidine-1-)pentanoate (compound 25):
[0063] Add intermediate 24 (400mg), 2-aminopyrimidine-5-boronic acid pinacol ester (250mg), Pd(PPh 3 ) 4 (119mg), saturated NaHCO 3 solution (2mL), DME (8mL), H 2 O (2 mL), the system was fully replaced with nitrogen, and stirred and reacted...
Embodiment 2
[0067] Synthesis of 5-(4-amino-3-(6-aminopyridine-3-)-1H-pyrazolo[3,4-d]pyrimidine-1)-N-hydroxypentanamide (Compound 1).
[0068] Compound 1 was prepared with reference to Example 1, only in step (2), 2-aminopyrimidine-5-boronic acid pinacol ester was replaced by 2-aminopyridine-5-boronic acid pinacol ester, and then the same method was used for hydroxylamine hydrolysis An off-white solid was obtained.
[0069] The result of high resolution mass spectrometry is: ESI-HRMS: 343.1639[M+H] + .
Embodiment 3
[0071]Synthesis of 7-(4-amino-3-(6-aminopyridine-3-)-1H-pyrazolo[3,4-d]pyrimidine-1)-N-hydroxyheptanamide (compound 2).
[0072] Compound 2 was prepared with reference to Example 1, and only in step (1) was 5-bromopentanoic acid ethyl ester replaced with 7-bromoheptanoic acid ethyl ester, and in step (2) 2-aminopyrimidine-5-boronic acid pina The alcohol ester was replaced by 2-aminopyridine-5-boronic acid pinacol ester, and then the off-white solid was obtained by hydroxylaminolysis using the same method.
[0073] 1 H NMR (400MHz, DMSO-d 6 ):δ8.22(s,1H),8.18(d,2.0Hz,1H),7.64(dd,2.0Hz,8.8Hz,1H),6.80(brs,2H),6.58(d,8.8Hz,1H) ,6.24(s,2H),4.29(t,6.8Hz,2H),1.92(t,7.6Hz,2H),1.84-1.75(m,2H),1.51-1.40(m,2H),1.26-1.24( m,4H); ESI-HRMS: 371.1949[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com