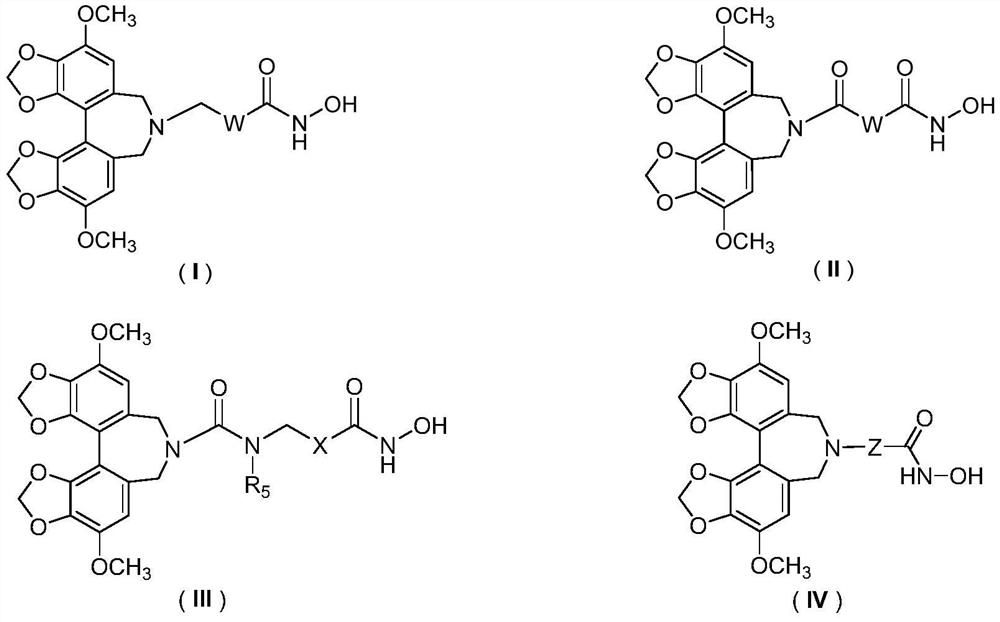
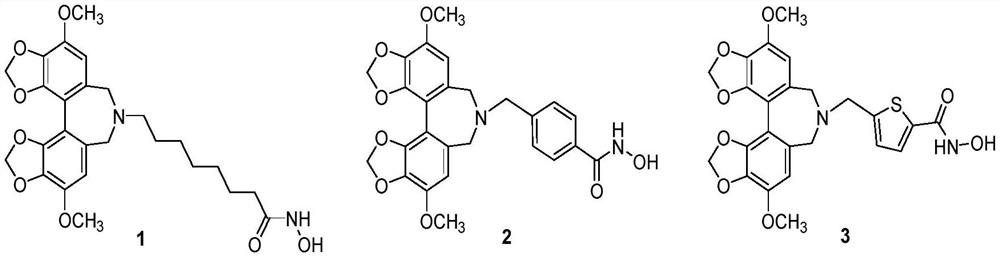
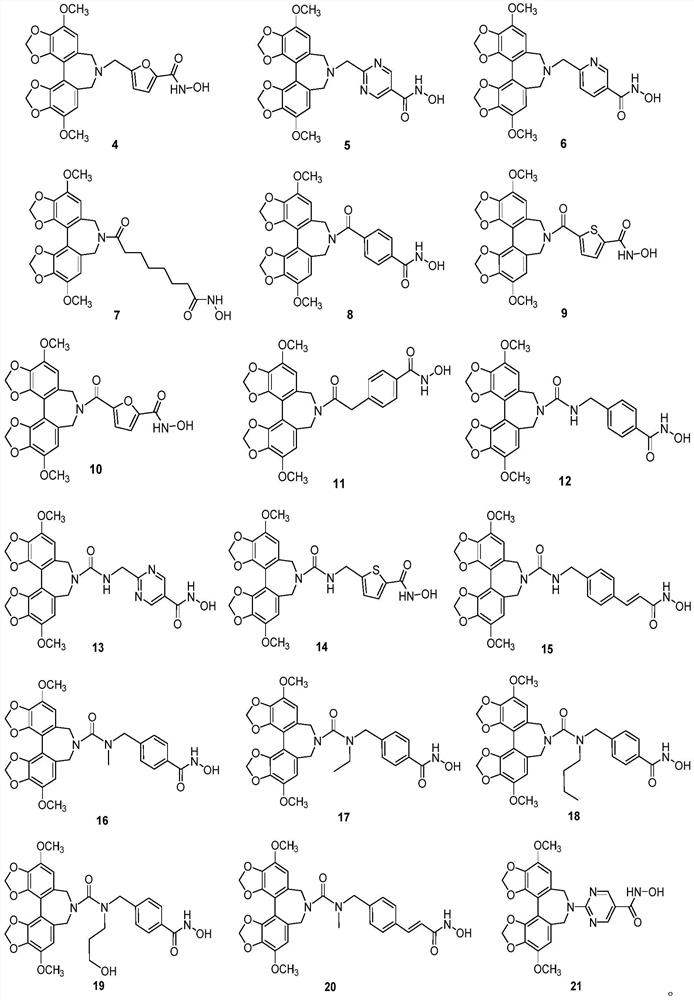
A class of hdac inhibitors and uses thereof
A technology of inhibitors and uses, applied in the field of bifendate derivatives and their preparation, can solve the problems of no HDAC inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 8-(4,10-Dimethoxy-6,8-dihydro-7H-[1,3]dioxol[4',5':3,4]benzo[1,2-c][ Synthesis of 1,3]dioxolo[4',5':5,6]benzo[1,2-e]azepine-7-)-N-hydroxyoctylamide (1)
[0061] (1), Synthesis of (7,7'-dimethoxy-[4,4'-dibenzo[d][1,3]dioxol]-5,5'-)dimethanol (23)
[0062]
[0063] Add bifendate (10.0g, 23.9mmol, 1eq) into a round bottom flask, dissolve it with anhydrous THF (150mL), add LiAlH in batches under ice-cooling 4 (2.72g, 71.7mmol, 3eq), after the addition was complete, it was transferred to room temperature for 4h. Under ice bath, add ice water to remove excess LiAlH 4 , suction filtration, DCM rinse, the filtrate was concentrated, followed by saturated NaHCO 3 Solution, water, saturated brine washing, anhydrous Na 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain 8.33 g of white solid.
[0064] (2), (7,7'-dimethoxy-[4,4'-dibenzo[d][1,3]dioxol]-5,5'-)bis(methylene)bis Synthesis of Mesylate (24)
[0065]
[0066] Intermediate 23 (5.5...
Embodiment 2
[0075] 4-((4,10-Dimethoxy-6,8-dihydro-7H-[1,3]dioxol[4',5':3,4]benzo[1,2-c] Synthesis of [1,3]dioxol[4',5':5,6]benzo[1,2-e]azepine-7-)methyl)-N-hydroxybenzamide (2)
[0076] (1), 7-benzyl-4,10-dimethoxy-7,8-dihydro-6H-[1,3]dioxol[4',5':3,4]benzo[1 ,2-c][1,3]Dioxol[4',5':5,6]Benzo[1,2-e]azepine (25) Synthesis
[0077]
[0078] Intermediate 24 (7.57g, 14.6mmol, 1eq), CH 3 CN (50mL), benzylamine (6.38mL, 58.4mmol, 4eq) and TEA (8.12mL, 58.4mmol, 4eq) were reacted at 40°C for 8h. spin off CH 3 CN, followed by saturated NaHCO 3 Solution, water, saturated brine washing, DCM extraction, anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure and recrystallized from ethyl acetate to obtain 5.01 g of white solid.
[0079] (2), 4,10-dimethoxy-7,8-dihydro-6H-[1,3]dioxol[4',5':3,4]benzo[1,2-c] Synthesis of [1,3]dioxin[4',5':5,6]benzo[1,2-e]azepine(26)
[0080]
[0081] Intermediate 25 (5.00g, 11.5mmol, 1eq), HCOONH 4 (4.00g, 63....
Embodiment 3
[0090] 5-(4,10-Dimethoxy-7,8-dihydro-6H-[1,3]dioxol[4',5':3,4]benzo[1,2-c][ Synthesis of 1,3]dioxol[4',5':5,6]benzo[1,2-e]azepine-7-carbonyl)-N-hydroxythiophene-2-carboxamide (9)
[0091] (1), 5-(4,10-dimethoxy-7,8-dihydro-6H-[1,3]dioxol[4',5':3,4]benzo[1,2 -c][1,3]dioxol[4',5':5,6]benzo[1,2-e]azepine-7-carbonyl)thiophene-2-carboxylic acid methyl ester (28) Synthesis
[0092]
[0093] 5-Methoxycarbonylthiophene-2-carboxylic acid (89.5mg, 0.481mmol, 1.1eq), EDC·HCl (92.1mg, 0.481mmol, 1.1eq), HOBt (65.4mg, 0.481mmol , 1.1eq), DIPEA (217μL, 1.31mmol, 3eq) and DCM (2mL), stirred at room temperature for 1h. Intermediate 26 (150mg, 0.437mmol, 1eq) was added, and the reaction was continued at room temperature for 4h. followed by saturated NaHCO 3 Solution, water, saturated brine washing, DCM extraction, anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure and subjected to silica gel column chromatography to obtain 208 mg of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com