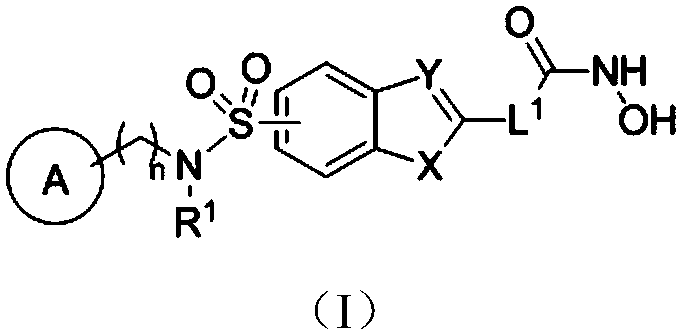
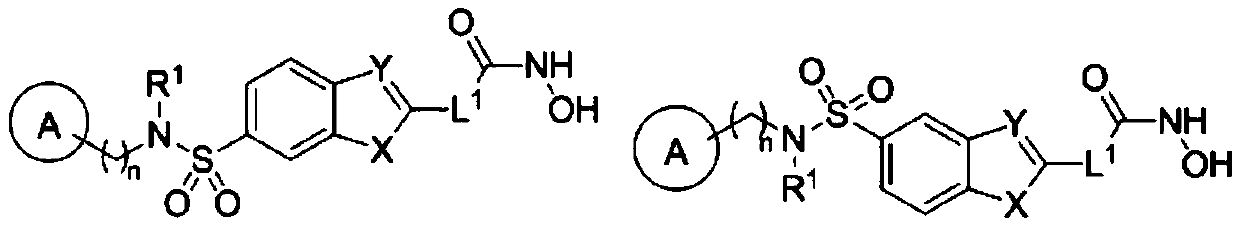
HDAC inhibitor as well as preparation method and application thereof
A CH2, solvate technology, applied in the field of HDAC inhibitors and its preparation and application, can solve the problem of subtype selectivity of anticancer activity toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] The synthesis of compound among the present invention in embodiment 1
[0124] General Synthetic Route 1
[0125]
[0126] (1.1) Synthesis of intermediate 3a:
[0127]
[0128] 4-Bromo-2-fluorobenzaldehyde (100g, 493mmol) and 2-methyl glycolate (102g, 1.13mol) were dissolved in DMF (1.0L), and sodium hydrogen (59.2g, 1.48mol) was added at 0°C , 60%), stirred for 1 hour, added ice water (2.0 L), extracted 4 times with ethyl acetate (1.0 L), combined the organic layers and washed 1 time with saturated sodium chloride solution. The solvent was evaporated under reduced pressure, and intermediate 3a (22.0 g, 86.3 mmol, yield 18%) was obtained after purification by column chromatography.
[0129] (1.2) Synthesis of intermediate 3b:
[0130] Using 5-bromo-2-fluorobenzaldehyde and methyl 2-hydroxyacetate as raw materials, according to the synthesis method of intermediate 3a, intermediate 3b was obtained (yield 42%).
[0131] (1.3) Synthesis of intermediate 3c:
[013...
Embodiment 2
[0195] Synthesis of compounds in embodiment 2 of the present invention
[0196] General synthetic route 2:
[0197]
[0198] (1.1) Synthesis of Intermediate 9a
[0199]
[0200] 5-Bromobenzo[d]thiazole (2.57g, 12.0mmol) was dissolved in dry tetrahydrofuran (75.0mL), and lithium hexamethyldisilazide (17.0mL) was added dropwise at -78°C. The reaction was stirred at -78°C for 30 minutes. Ethyl cyanoformate (1.80 g, 18.2 mmol) was added dropwise and the reaction was continued to stir at -78°C for 30 minutes. After the reaction was completed, it was quenched by adding saturated ammonium chloride solution (50.0 mL) and warmed to room temperature. Ethyl acetate (30 mL) was extracted three times, the organic layers were combined, the solvent was evaporated under reduced pressure, and the intermediate 9a was purified by column chromatography (660 mg, 2.31 mmol, yield 19%).
[0201] (1.2) Synthesis of Intermediate 9b
[0202] Using 6-bromobenzo[d]thiazole and ethyl cyanoforma...
Embodiment 3
[0226] Embodiment 3, the preparation of compound of the present invention
[0227] General synthetic route 3:
[0228]
[0229] (1.1) Synthesis of intermediate 14a
[0230] Starting from methyl 5-bromo-1H-indole-2-carboxylate, according to the synthesis method of intermediate 4a, intermediate 14a was obtained (60% yield).
[0231] (1.2) Synthesis of intermediate 14b
[0232] Starting from methyl 6-bromo-1H-indole-2-carboxylate, according to the synthesis method of intermediate 4a, intermediate 14b was obtained (yield 72%).
[0233] (1.3) Synthesis of Intermediate 14c
[0234] Starting from ethyl 5-bromo-1-methyl-1H-indole-2-carboxylate, according to the synthesis method of intermediate 4a, intermediate 14c was obtained (yield 71%).
[0235] (1.4) Synthesis of intermediates 15a2-15a24
[0236] The raw materials in the following table were used to obtain intermediates 15a1 to 15a24 according to the synthesis method of intermediate 6a1.
[0237]
[0238]
[0239] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com