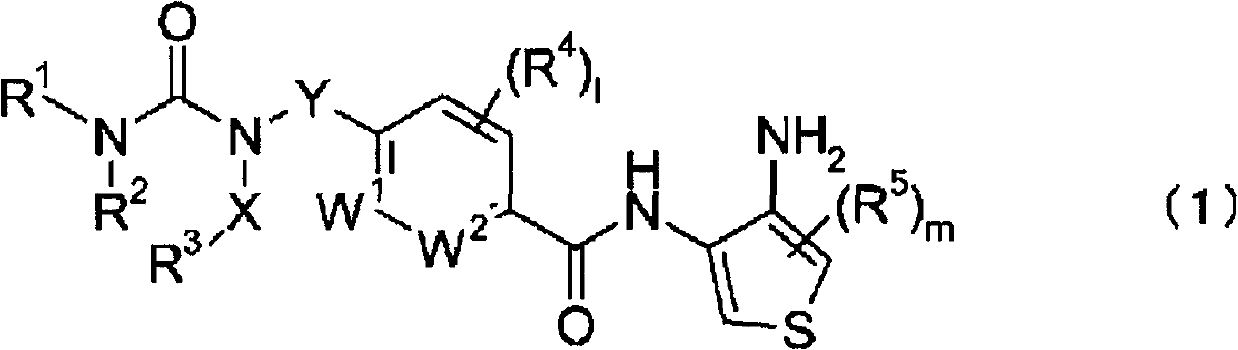
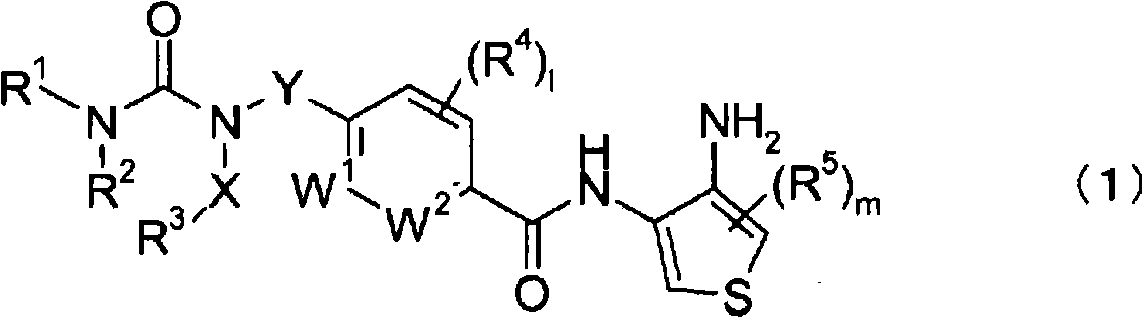
Novel thiophenediamine derivative having urea structure
A compound, low-level technology, applied in the directions of medical preparations containing active ingredients, organic chemistry, drug combinations, etc., to achieve the effect of changing the shape of trabecular cells and reducing intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0165] The production method of the compound of the present invention can be roughly classified into the methods shown below, and the method can be appropriately selected according to the type of the substituent.
[0166] 1) Compound (I) of the present invention can be prepared according to Synthetic Route 1. That is, the compound (I) of the present invention can be obtained by treating the compound (II) in an organic solvent such as methanol in the presence of an acid such as hydrogen chloride-ethyl acetate at 0°C to room temperature for 30 minutes to 24 hours.
[0167] Synthetic pathway 1
[0168]
[0169] Compound (IIa, R 2 =H) can be prepared according to synthetic route 1-1. That is, the compound can be obtained by reacting compound (III) and isocyanate (IV) in an organic solvent such as dichloromethane at 0° C. to room temperature for 30 minutes to 24 hours.
[0170] Synthetic pathway 1-1
[0171]
[0172] Compound (III) can be prepared according to Synthetic R...
reference example 1
[0200] 3-amino-4-tert-butoxycarbonylaminothiophene (reference compound 1-1)
[0201] Under ice cooling, di-tert-butyl dicarbonate ( 0.16g, 0.72mmol), stirred overnight at room temperature. Water (40 mL) was added to the reaction liquid, followed by extraction with ethyl acetate (30 mL, twice). The organic layer was washed with water (30 mL) and saturated brine (30 mL), dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The obtained residue was purified by silica gel column chromatography (hexane-ethyl acetate) to obtain the title reference compound (87 mg) as a brown solid. (Yield 58%)
[0202] [Table 1]
[0203]
reference example 2
[0205] N-(4-tert-butoxycarbonylaminothiophen-3-yl)-5-methoxycarbonylpyridine-2-carboxamide (reference compound 2-1)
[0206] To 3-amino-4-tert-butoxycarbonylaminothiophene (reference compound 1-1, 0.68g, 3.2mmol), 5-methoxycarbonylpyridine-2-carboxylic acid (0.64g, 3.5mmol) and N-form HATU (1.3 g, 3.5 mmol) was added to a solution of morpholine (0.70 mL, 6.4 mmol) in DMF (20 mL), and stirred overnight at room temperature. Water (0.30 L) and ethyl acetate (0.40 L) were added, and the insoluble matter was filtered to obtain the title reference compound (0.50 g) as a pale yellow solid. Furthermore, the filtrate was separated into layers, and the organic layer was dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the precipitated solid was filtered with ethyl acetate (30 mL) to obtain the title reference compound (0.30 g) as a pale yellow solid. (Yield 67%)
[0207] [Table 2]
[0208]
[0209] Hereinafter, reference compound 2-2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com