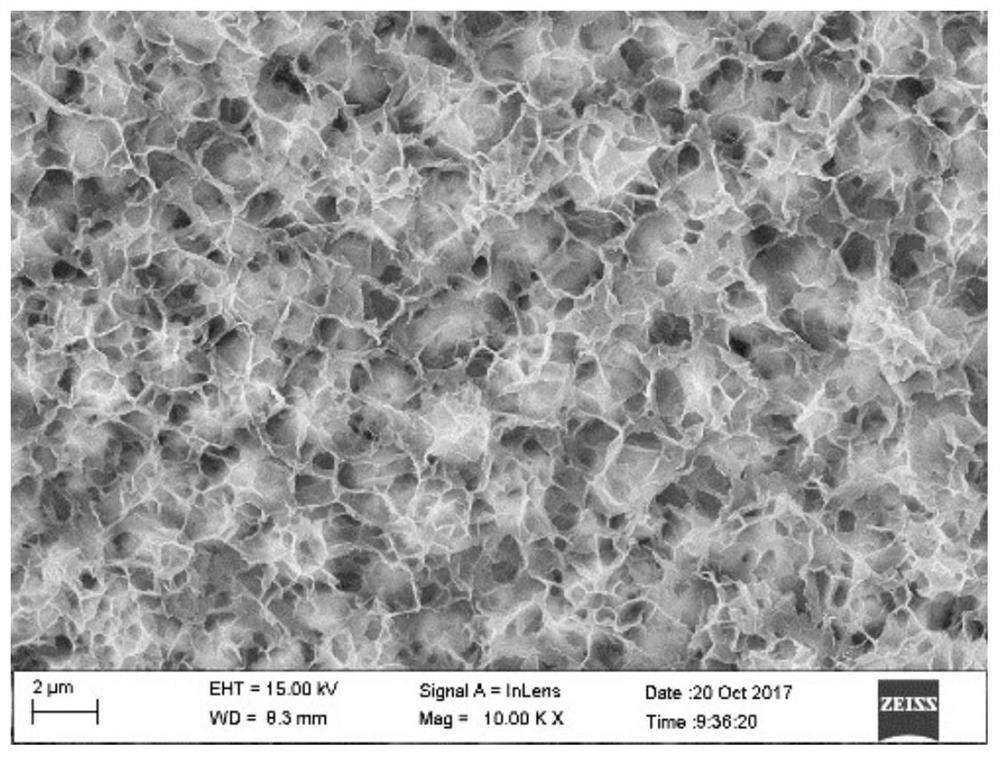
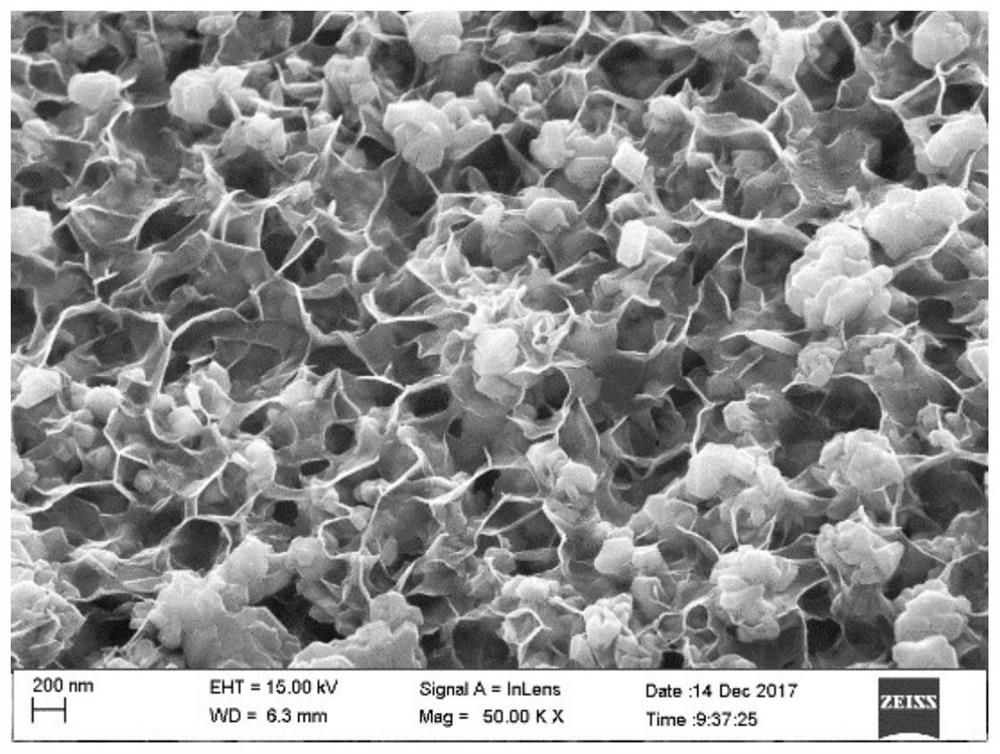
A lithium carbon dioxide battery pole piece without a binder and without a conductive agent and its preparation method
A battery pole piece, binder-free technology, applied in non-aqueous electrolyte battery electrodes, battery electrodes, circuits, etc., can solve the problems of application limitation, increase the complexity of the process, increase the production cost, etc., to shorten the process flow, reduce Human and material cost, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] This embodiment proposes a method for preparing a lithium carbon dioxide battery pole piece without a binder and without a conductive agent, including the following steps:
[0031] S1, place the nickel foam that has removed the surface oxide layer in the copper salt solution, and then clean and dry;
[0032] S2. Place the dried nickel foam in a mixed solution for hydrothermal reaction, then take it out, wash it, and dry it to obtain a battery pole piece precursor;
[0033] The mixed solution contains copper ions, nickel ions and urea;
[0034] S3. Place the battery pole piece precursor in a 2- react in the solution, and then take it out, wash it, and dry it to obtain a battery pole piece.
[0035] In this embodiment, the concentration of copper ions in the copper salt solution is 0.002 mol / L˜0.2 mol / L; the copper salt solution includes one of copper nitrate, copper sulfate and copper chloride.
[0036] In this embodiment, the concentration of copper ions in the mixed...
Embodiment 1
[0049] S1. Add 0.232 g of copper nitrate trihydrate into 120 ml of deionized water, and stir evenly to obtain a copper nitrate solution. Soak 2*12*0.1cm of nickel foam from which the surface oxide layer has been removed in copper nitrate solution for 0.5 min, so that copper nitrate and nickel foam undergo an alloy reaction to form a nickel-copper alloy on the surface of nickel foam.
[0050] Take out the soaked nickel foam, wash it in an ultrasonic cleaner filled with ultrapure water for 5 minutes, and then place it in a vacuum oven at 60°C until it is completely dry.
[0051] S2. Add 0.232g of copper nitrate trihydrate and 0.14g of nickel nitrate hexahydrate into 120ml of deionized water, stir evenly, then add 0.086g of urea, stir evenly to obtain a mixed solution.
[0052] The nickel foam dried in step S1 and the mixed solution were placed together in a 200 mL stainless steel reactor for hydrothermal reaction.
[0053] Preferably, the reactor is placed in a thermostat at 12...
Embodiment 2
[0059] S1. Add 0.3 g of copper nitrate trihydrate into 120 ml of deionized water, and stir evenly to obtain a copper nitrate solution. Soak 2*12*0.1cm of nickel foam from which the surface oxide layer has been removed in copper nitrate solution for 3 minutes, so that copper nitrate and nickel foam undergo an alloy reaction to form a nickel-copper alloy on the surface of nickel foam.
[0060] Take out the soaked nickel foam, wash it in an ultrasonic cleaner filled with ultrapure water for 5 minutes, and then place it in a vacuum oven at 60°C until it is completely dry.
[0061] S2. Add 0.3 g of copper nitrate trihydrate and 0.14 g of nickel nitrate hexahydrate into 120 ml of deionized water, stir evenly, then add 0.2 g of urea, stir evenly to obtain a mixed solution.
[0062] The nickel foam dried in step S1 and the mixed solution were placed together in a 200 mL stainless steel reactor for hydrothermal reaction.
[0063] Preferably, the reactor is placed in a thermostat at 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com