Catalyst for the preparation of 1,3-butadiene from butene
A catalyst and butadiene technology, applied in the chemical industry, can solve the problems of uneven distribution of active centers, reduced anti-wear performance of catalysts, loss of active metals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
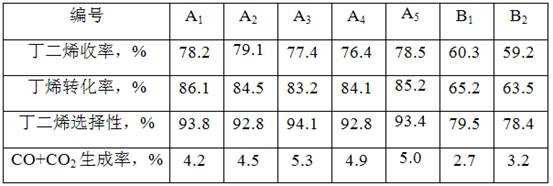
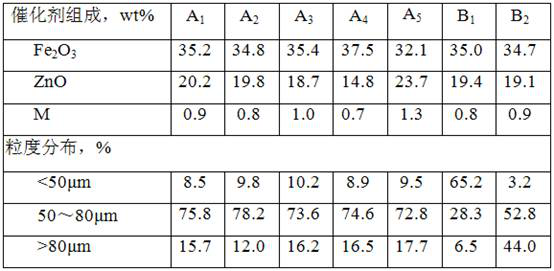
[0037] (1) Weigh 174.6g Fe(NO 3 ) 2 . 9H 2 O, 144.2g Zn(NO 3 ) 2 . 6H 2 O, 5.2g Cr(NO 3 ) 3 . 9H 2 0 and 7g polyethylene glycol, mixed with 200mL solution;
[0038] (2) Weigh 100g of self-made alumina carrier (specific surface 298, pore volume 0.93);
[0039] (3) Weigh 95mL solution to impregnate the alumina carrier for one stage. After impregnation, the catalyst is dried at 120°C for 4 hours, then weigh 62mL solution for two stage impregnation. After impregnation, the catalyst is dried at 115°C for 5 hours, and finally Weigh 41mL solution for three-stage impregnation. After impregnation, the catalyst is dried at 115°C for 5 hours; after drying, it is roasted, wherein the temperature is raised to 140°C at a heating rate of 2.0°C / min, and the heating rate of 140~170°C is 1.3 ℃ / min, the heating rate of 170~210℃ is 0.7℃ / min, the heating rate of 210~270℃ is 3.0℃ / min, the heating rate of 270~600℃ is 2.0℃ / min, the temperature is kept at 600℃ for 3 hours, and the catalyst...
Embodiment 2
[0041] Other conditions are the same as embodiment 1, just change the Polyethylene Glycol quality into 8g in the preparation solution, 5.2g Cr(NO 3 ) 3 . 9H 2 O was changed to 11.2Mg(NO 3 ) 2 . 6H 2 O, during the catalyst calcination process, the heating rate was raised to 140°C at a rate of 1.8°C / min, the heating rate was 1.2°C / min at 140-165°C, the heating rate at 165-215°C was 0.6°C / min, and the heating rate at 225-265°C is 2.8°C / min, and the heating rate is 1.8°C / min from 265 to 600°C to obtain catalyst A 2 , and its physical and chemical properties are listed in Table 1.
Embodiment 3
[0043] Other conditions are the same as embodiment 1, just change the Polyethylene Glycol quality into 6g in the preparation solution, 5.2g Cr(NO 3 ) 3 . 9H 2 O was changed to 6.51Mn(NO 3 ) 2 . 6H 2 O, during the catalyst calcination process, the heating rate was raised to 140 °C at a rate of 2.1 °C / min, the heating rate was 1.4 °C / min at 140-175 °C, the heating rate at 175-205 °C was 0.8 °C / min, and the heating rate at 205-275 °C is 3.2°C / min, and the heating rate is 2.1°C / min from 275 to 600°C to obtain catalyst A 3 , and its physical and chemical properties are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com