Halo-substituted piperidines as orexin receptor modulators
A halogen and substituent technology, applied in anti-inflammatory agents, metabolic diseases, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0423] Embodiment 1: synthetic scheme
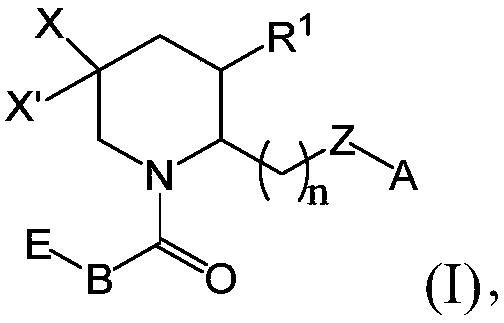
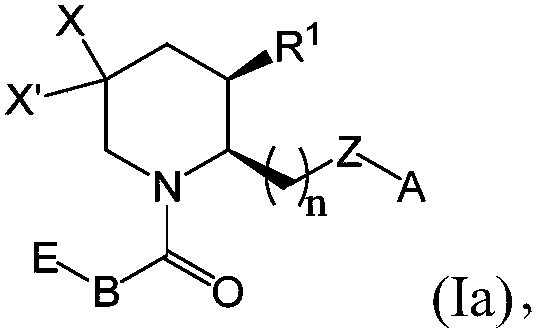
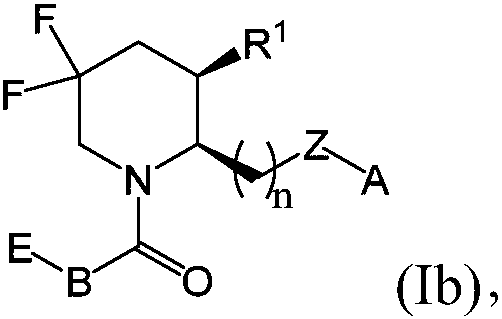
[0424] Exemplary chemical entities useful in the methods of the present application will now be described by reference to the illustrative synthetic schemes for the general preparations below and the specific examples that follow. The skilled artisan will recognize that in order to obtain the various compounds of the present application, the starting materials can be appropriately selected such that the final desired substituents are carried through the reaction schemes, with or without protection, as appropriate, to produce the desired desired product. Alternatively, it may be necessary or desirable to use an appropriate group in place of the final desired substituent, which group can be worked through the reaction scheme and replaced with the desired substituent as appropriate. Furthermore, those skilled in the art will recognize that the permutations shown in the schemes below can be performed in any order compatible with the funct...
Embodiment 2
[1214] Example 2: Functional Assay Based on Orexin Receptor Cells
[1215] Measured using FLIPR [Ca 2+ ]i: In F12-K medium supplemented with 10% FBS, CHO-OX 1 or CHO-OX 2 Cells were seeded into black-walled transparent-bottom 384-well plates (Corning, catalog number 3712) at a density of 20,000 cells / well, and then incubated 2 , Incubate overnight in a 37°C incubator to reach 90% confluence. Cells were incubated with an equal volume of Calcium 6 loading buffer (Molecular Devices, Inc.) containing 2.5 mM probenecid for 2 hours at 37°C and then re-incubated with test compounds (dose range 0.1 nM-10 μM). Incubate for 30 minutes. The plate was then placed in FLIPR (Molecular Devices, Inc.) to react with [OX]-added EC 90 Fluorescence was monitored before and after (λ excitation 488 nm, lambda emission 540 nm). Results for Exemplary Compounds of Formula (I), (Ia), (Ib), (II), (IIa), (III), (IIIa), (IV), (IVa), (V) or (Va) Shown in Table 3.
[1216] Table 3: Exemplary compoun...
Embodiment 3
[1223] Example 3: Nicotine Self-Administration Assay
[1224] For all experiments, rats weighing 250-300 g were housed in groups of 1-23 / cage in a temperature-controlled vivarium on a reversed 12-h light / dark cycle (lights off at 8 a.m. ) feeding. Food and water were provided ad libitum until initiation of behavioral training. During training, rats were food-restricted to maintain approximately 85-90% of their body weight on free-feeding. Behavioral testing was performed during the dark portion of the light / dark cycle between 9 am and 1 pm during the early phase of the dark phase of the cycle. All operations were performed in strict compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. Rats were anesthetized by inhalation of 1-3% isoflurane / oxygen, and a silicone rubber catheter was inserted into the jugular vein. Briefly, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com