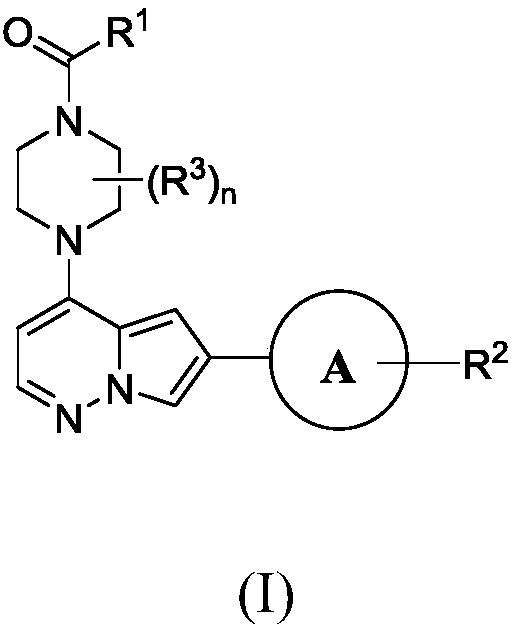
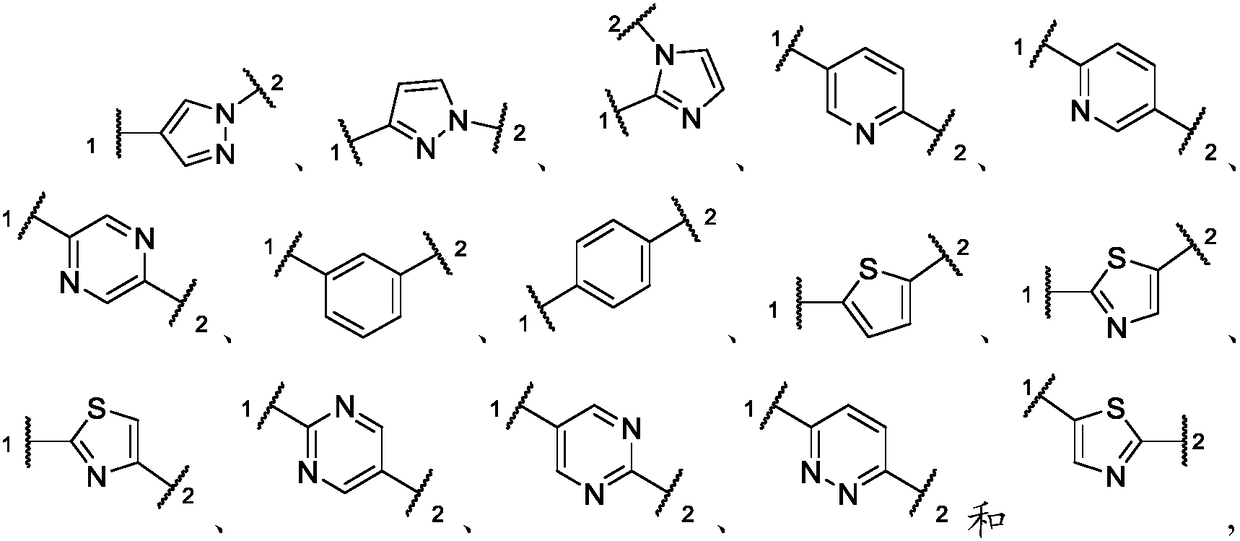
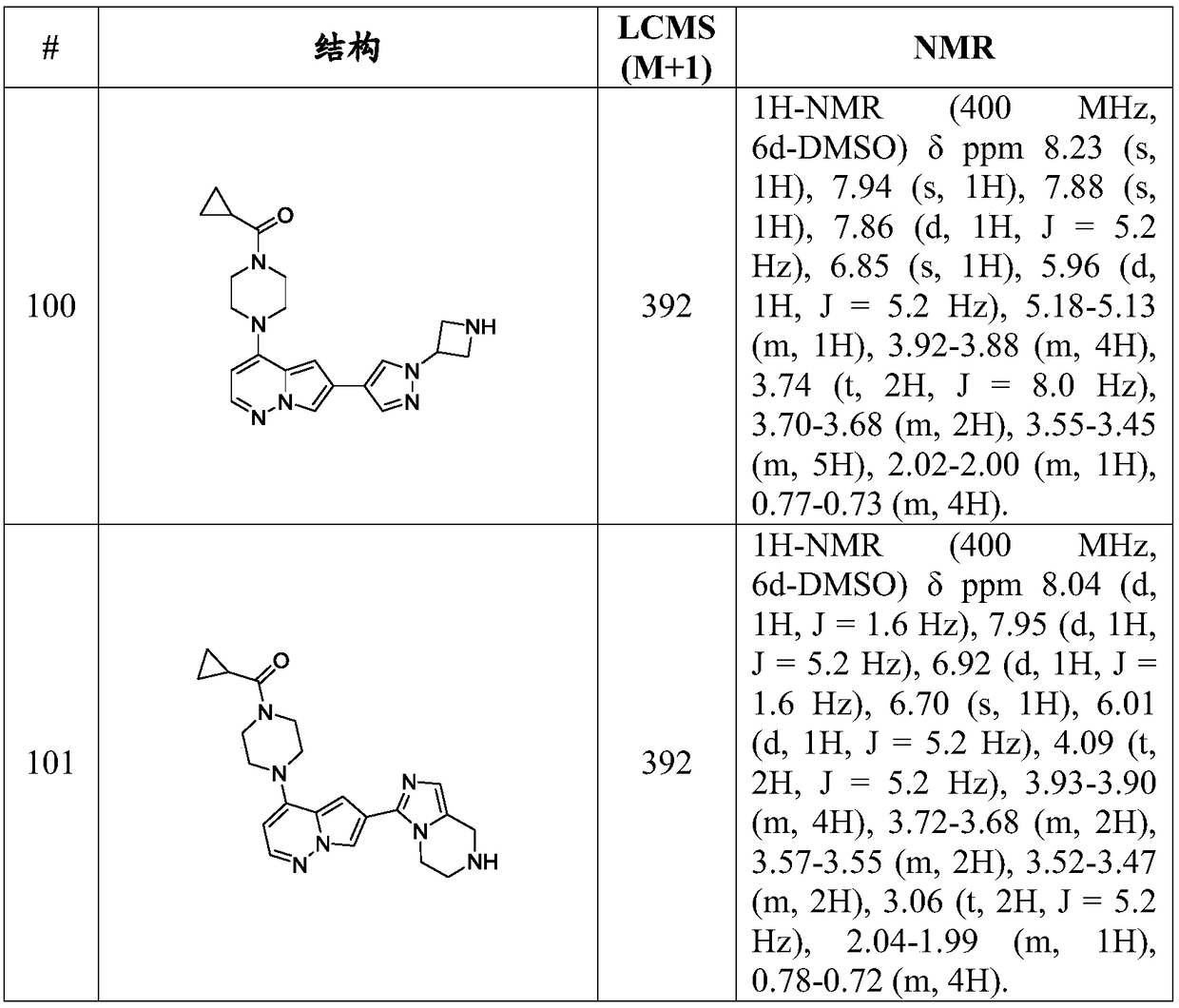
Inhibitors of activin receptor-like kinase
A technology of alkyl and heterocyclic groups, applied in the field of pharmaceutical compositions, can solve problems that hinder the identification of new treatment options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0549] Example 1. Synthesis of 6-bromopyrrolo[1,2-b]pyridazin-4-yl trifluoromethanesulfonate
[0550] Step 1: Synthesis of 1-(4-bromo-1H-pyrrol-2-yl)ethanone (Intermediate B)
[0551]
[0552] Amberlyst 15 (0.09 g / g-bulk-LR) was added to commercially available 1-(1H-pyrrol-2-yl)ethanone (70 g; 1.00 equiv; 641.45 mmol) in tetrahydrofuran (10 mL / g-bulk-LR; 9.71 moles; 700.00 mL; 700.00 g). Next, 1-bromopyrrolidine-2,5-dione (1 equivalent (molar); 641.45 mmol; 114.17 g) was added in portions at -30 to -20°C, and stirred for about 1 h until LCMS showed that the reaction was complete. The reaction mixture was then filtered, and the filtrate was washed with saturated Na 2 SO 3 The aqueous solution (350 mL) was quenched and extracted with DCM (700 mL x 2). The organic layer was concentrated, then diluted with MTBE (700 mL). The organic layers were combined, followed by saturated NaHCO 3 (350 mL x 2) washed, concentrated by rotary evaporator under reduced pressure to give 1...
Embodiment 2
[0577] Example 2. Synthesis of cyclopropyl (4-(6-(4-(piperazin-1-yl)phenyl)pyrrolo[1,2-b]pyridazin-4-yl)piper azin-1-yl)methanone (Compound 127).
[0578] Step 1: Synthesis of (4-(6-bromopyrrolo[1,2-b]pyridazin-4-yl)piperazin-1-yl)(cyclopropyl)methanone.
[0579]
[0580] 6-bromopyrrolo[1,2-b]pyridazin-4-yl trifluoromethanesulfonate (30g, 86.9mmol), cyclopropyl(piperazin-1-yl)methanone (16.0g, 104mmol ) and triethylamine (13.1 g, 130 mmol) in NMP (300 mL) was stirred at 100° C. for 30 min. The reaction mixture was cooled and diluted with EA. The organic layer was washed with water and brine, concentrated, and purified by silica gel column to give the title product (26.0 g, yield 86%) as a yellow solid. MS(ES+)C 15 h 17 BrN 4 O required value: 348, measured value: 349 [M+H] + .
[0581] Step 2: Synthesis of cyclopropyl(4-(6-(4-(piperazin-1-yl)phenyl)pyrrolo[1,2-b]pyridazin-4-yl)piperazin-1-yl) Methanone (compound 127).
[0582]
[0583] (4-(6-Bromopyrrolo[1,2...
Embodiment 3
[0584] Example 3. Synthesis of cyclopropyl (4-(6-(4-(4-(2-hydroxyethyl)piperazin-1-yl)phenyl)pyrrolo[1,2- b] pyridazin-4-yl)piperazin-1-yl)methanone (compound 274)
[0585]
[0586] Cyclopropyl (4-(6-(4-(piperazin-1-yl)phenyl)pyrrolo[1,2-b]pyridazin-4-yl)piperazin-1-yl)methanone ( A mixture of 100 mg, 232 μmol), 2-bromoethanol (57.9 mg, 464 μmol) and potassium carbonate (32 mg, 0.232 mmol) was stirred overnight (~12h) at 70°C. The reaction mixture was cooled and concentrated. The residue was purified by prep-HPLC to afford the title compound (10.5 mg, yield 9.5%) as a white solid. MS(ES+)C 27 h 34 N 6 o 2 , desired value: 474, measured value: 475 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com