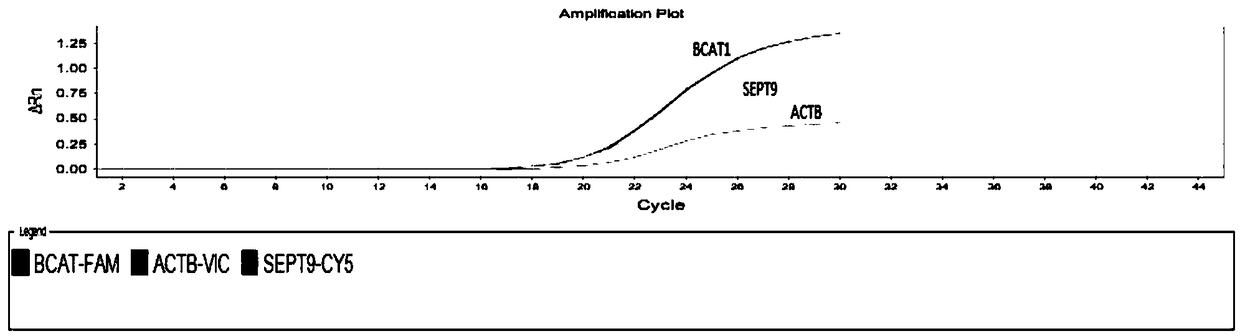
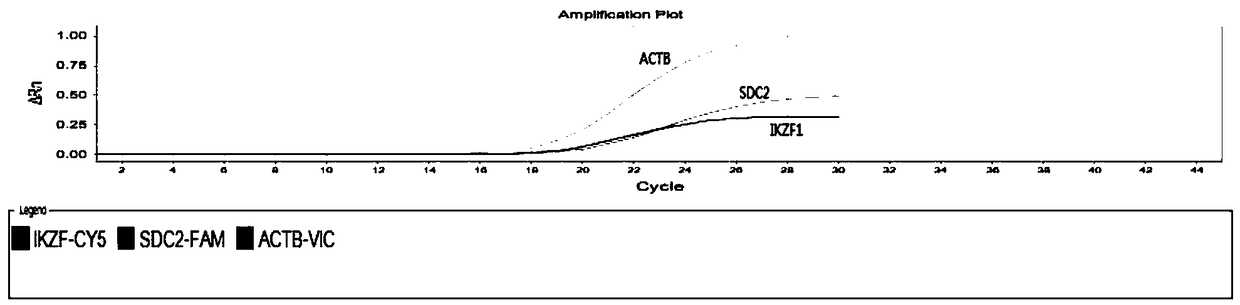
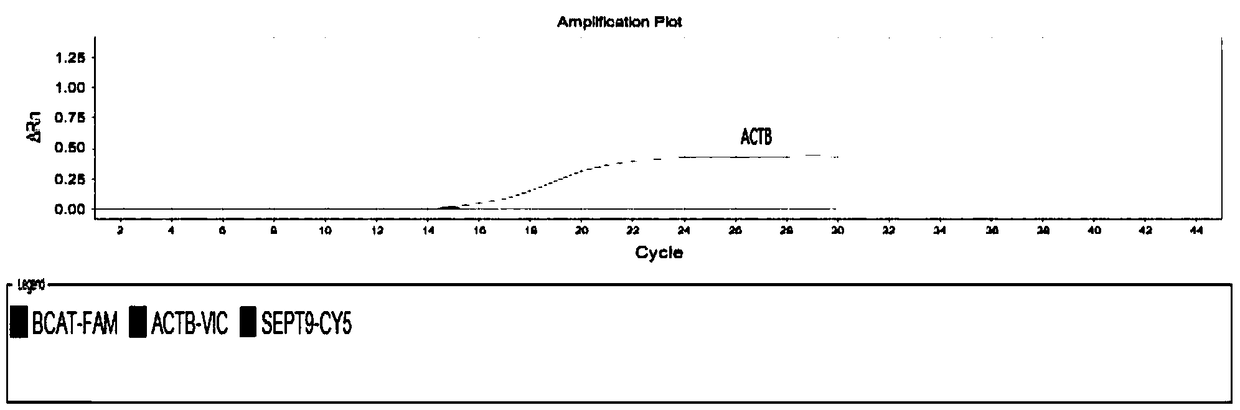
Kit used for detecting colorectal cancer and application of kit
A colorectal cancer and kit technology, applied in the field of oncology biological detection, can solve the problems of low detection sensitivity and large blood volume, and achieve the effects of high detection sensitivity, low false positive rate and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 is used to detect the kit composition of colorectal cancer
[0033] The composition of the kit for detecting colorectal cancer in this application can include as shown in Table 1:
[0034] Table 1 Composition of the kit
[0035]
Embodiment 2
[0036] Embodiment 2 is used to detect the kit composition of colorectal cancer
[0037] The composition of the kit for detecting colorectal cancer in this application can include as shown in Table 2:
[0038] Table 2 Composition of the kit
[0039]
[0040] The various parts used in Embodiment 1 and Embodiment 2 will be described in detail below:
[0041] Plasma free DNA modification reagents include: (1) bisulfite modification reagent contains 50 g of sodium bisulfite solid, which needs to be dissolved in 1 ml of deionized water; (2) DNA protection solution can contain water-soluble sodium bisulfite dissolved in dimethyl ether Sexual VE, its concentration can be 2-4M. Wherein, in some embodiments, the optimal concentration is 3.2M; (3) the binding solution may include guanidine isosulfate, the concentration of which is 5-8M. Wherein, in some embodiments, the optimum concentration may be 6.6M; (4) the desulfurization solution may include sodium hydroxide, and its concent...
Embodiment 3
[0052] The detection method of embodiment 3 kits of the present invention
[0053] 1) Carry out nucleic acid enrichment for the samples to be tested and quality control products, modify the eluent with bisulfite after elution, and obtain sample templates and quality control product templates after purification;
[0054] 2) Carry out PCR detection on the template of the sample to be tested and the template of the quality control product, and add the template of the sample to be tested and the template of the quality control product into two sets of PCR detection tubes;
[0055] 3) Dividing the sample template to be tested and the quality control template into detection tube I and detection tube II;
[0056] The composition of detection tube I is shown in Table 5:
[0057] Table 5 Composition of detection tube I
[0058] PCR master mix
15μl
PCR Detection Nucleotide Master Mix I
1μl
wxya 2 o
6μl
8μl
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com