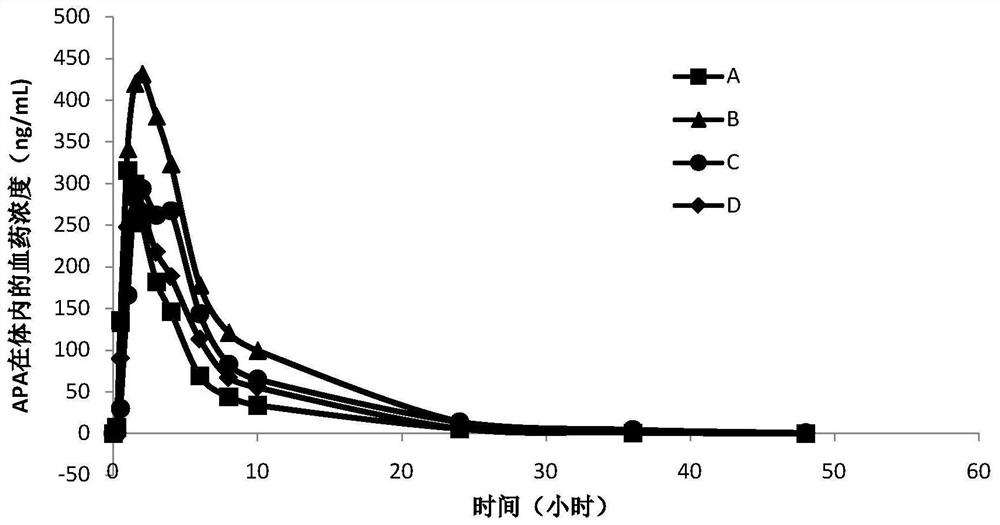
A kind of pharmaceutical composition of vegfr inhibitor and preparation method thereof
A composition and drug technology, which is applied in the direction of drug combination, pharmaceutical formula, antineoplastic drugs, etc., can solve the problems of individual differences in nano-preparations without literature reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1: Preparation and characterization of nanosuspension
[0051] Weigh about 2.5g of HPMC, add 100ml of purified water, stir to disperse and gradually dissolve, add 0.25g of SDS, stir to dissolve for later use;
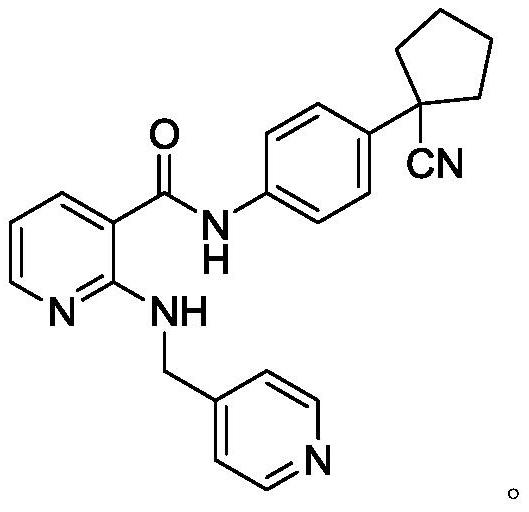
[0052] Weigh about 1.0g of N-[4-(1-cyanocyclopentyl)phenyl]-2-(4-pyridylmethyl)amino-3-pyridinecarboxamide (Compound A) and add it to 10ml of the aforementioned solution, stir Mix for 30 minutes, the approximate concentration of compound A is 100mg / ml;
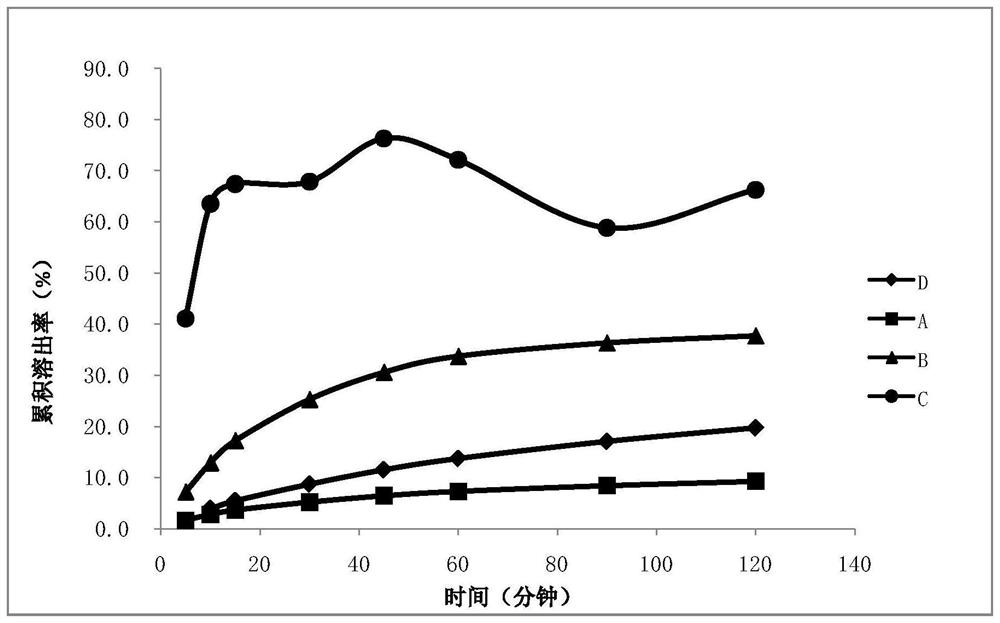
[0053]Use a 50ml ball mill tank, add small balls with a volume of 20ml, grind at a speed of 150rpm, grind for 60s, stop for 30s, before grinding (0h), after grinding 10min, 30min, 1h, 90min, 90min+10min (300rpm) , take a proper amount of samples with a syringe, and investigate the particle size (take the nanosuspension before grinding (0h) and grind for different times to directly detect the particle size), see Table 1.
[0054] Table 1: Particle size results before grinding and after grinding for di...
experiment example 1
[0056] Experimental Example 1: Physical Stability of Nanosuspension
[0057] Test 1: Take an appropriate amount of nano-suspension, seal it, place it at room temperature for 17 hours, and place it in a stability test chamber at 40°C for 6 hours to investigate the change in particle size of the suspension. It was found that after being placed at 40 degrees, D90 increased, D10 and D50 did not change significantly, and the stability at room temperature was better. The data are shown in Table 2.
[0058] Table 2:
[0059]
Embodiment 2
[0060] Embodiment 2: Nanoparticle tablet preparation
[0061]
[0062] 1) Preparation of nanosuspension
[0063] Weigh about 2.5g of HPMC (hydroxypropyl methylcellulose), 0.25g of SDS (sodium dodecyl sulfate), add 120ml of purified water, stir and dissolve for later use;
[0064] Weigh about 16.4mg of N-[4-(1-cyanocyclopentyl)phenyl]-2-(4-pyridylmethyl)amino-3-pyridinecarboxamide (compound A) and add it to 82ml of the aforementioned solution, Stir and mix well, the approximate concentration of compound A is 200mg / ml;
[0065] Using a 500ml ball mill tank, add 120ml volume of small balls and grind (grinding speed about 250rpm) to the target particle size. The particle size after grinding is shown in Table 3
[0066] table 3
[0067] sample D10 D50 D90 D[4,3] Compound A 45.5μm 108μm 207μm 77.5μm Compound A after milling 0.025μm 0.105μm 1.64μm 3.26μm
[0068] 2) Granulation
[0069] Take 25g of MCC PH101 in the granulator, turn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com