Degradable medical hemostatic material and preparation method thereof
A hemostatic material and degradable technology, which is applied in the field of degradable medical hemostatic materials and their preparation, can solve the problems affecting the hemostatic performance, porosity, small specific surface area, and wide distribution range of fiber diameters of gelatin hemostatic materials, so as to improve hemostasis Performance, good biocompatibility, and the effect of improving the speed of hemostasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
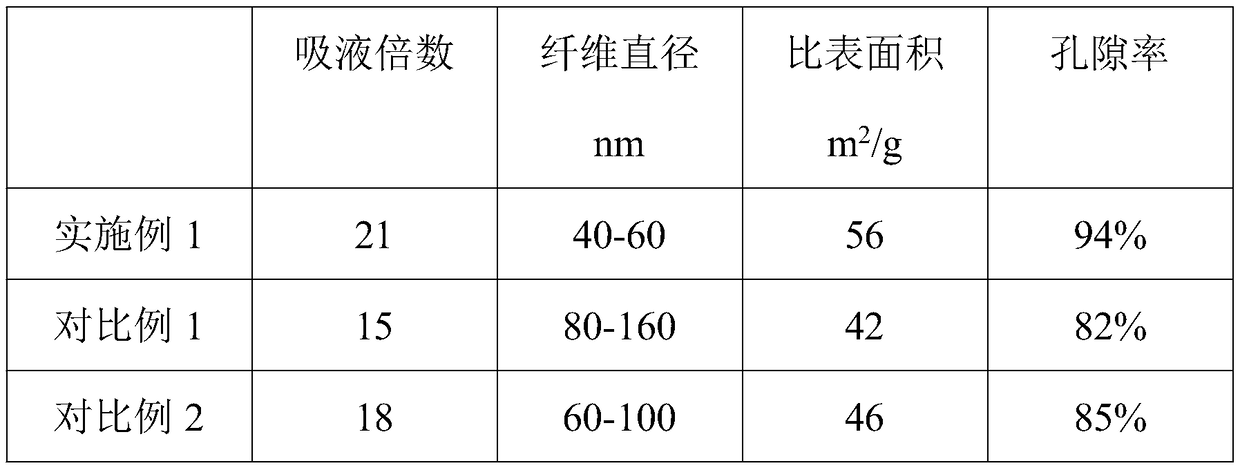
Embodiment 1
[0026] A preparation method of a degradable medical hemostatic material, comprising the steps of:
[0027] (1) Preparation of mixed solvent: mix polyethylene glycol, hyaluronic acid and deionized water in a volume ratio of 1:2:10;
[0028] (2) Divide the solution of step (1) into the same three parts, then respectively add gelatin, polylactic acid and chitosan to the three parts of solvent, and stir respectively for 3 hours at a temperature of 40° C. The solutions were mixed and then stirred and ultrasonicated for 1.5 hours; wherein, the mass concentrations of gelatin, polylactic acid and chitosan in the three solutions were 8%, 1%, and 0.5% respectively;
[0029] (3) The gelatin / polylactic acid / chitosan composite superfine fiber was prepared by electrospinning the mixed solution obtained in step (2), and dried in vacuum at 80° C. for 12 hours for subsequent use; the injection rate of electrospinning was 60 μL / min, the voltage is 22KV, and the distance between the spinneret ...
Embodiment 2
[0032] A preparation method of a degradable medical hemostatic material, comprising the steps of:
[0033] (1) Preparation of mixed solvent: mix polyethylene glycol, hyaluronic acid and deionized water in a volume ratio of 2:5:15;
[0034] (2) Divide the solution of step (1) into the same three parts, then respectively add gelatin, polylactic acid and chitosan to the three parts of solvent, and stir respectively for 3 hours at a temperature of 40° C. The solutions were mixed and then stirred and ultrasonicated for 1.5 hours; wherein, the mass concentrations of gelatin, polylactic acid and chitosan in the three solutions were 7%, 1%, and 0.5% respectively;
[0035] (3) The gelatin / polylactic acid / chitosan composite microfiber was prepared by electrospinning the mixed solution obtained in step (2), and dried in vacuum at 80° C. for 12 hours for subsequent use; the injection rate of electrospinning was 60 μL / min, the voltage is 22KV, and the distance between the spinneret and t...
Embodiment 3
[0038] A preparation method of a degradable medical hemostatic material, comprising the steps of:
[0039] (1) Preparation of mixed solvent: mix polyethylene glycol, hyaluronic acid and deionized water in a volume ratio of 1:4:12;
[0040] (2) Divide the solution of step (1) into the same three parts, then respectively add gelatin, polylactic acid and chitosan to the three parts of solvent, and stir for 3 hours respectively at a temperature of 40°C, and then divide the three parts The solutions were mixed and then stirred and ultrasonicated for 1.5 hours; wherein, the mass concentrations of gelatin, polylactic acid and chitosan in the three solutions were 10%, 1%, and 0.5% respectively;
[0041] (3) The gelatin / polylactic acid / chitosan composite superfine fiber was prepared by electrospinning the mixed solution obtained in step (2), and dried in vacuum at 80° C. for 12 hours for subsequent use; the injection rate of electrospinning was 80 μL / min, the voltage is 22KV, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com