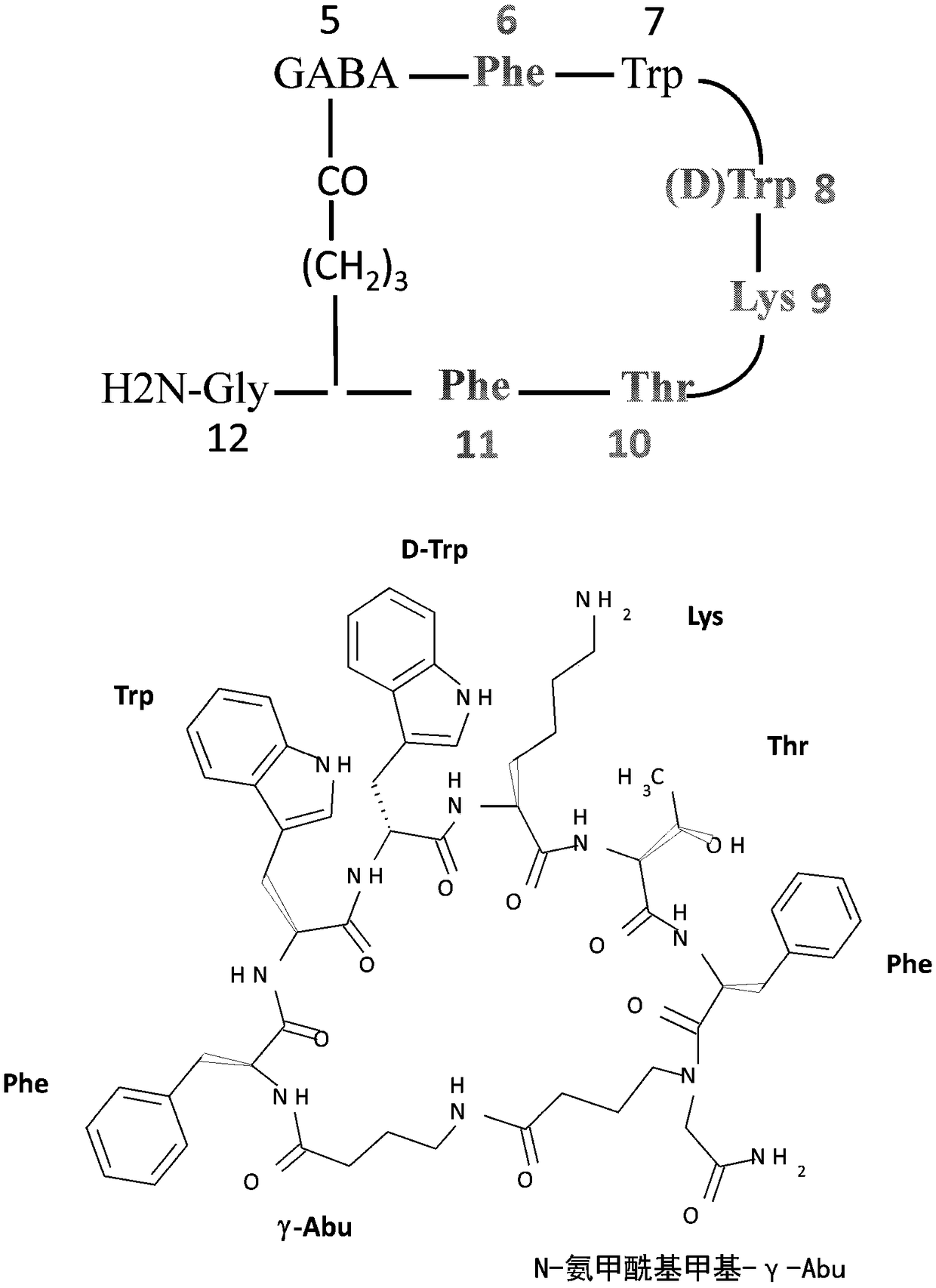
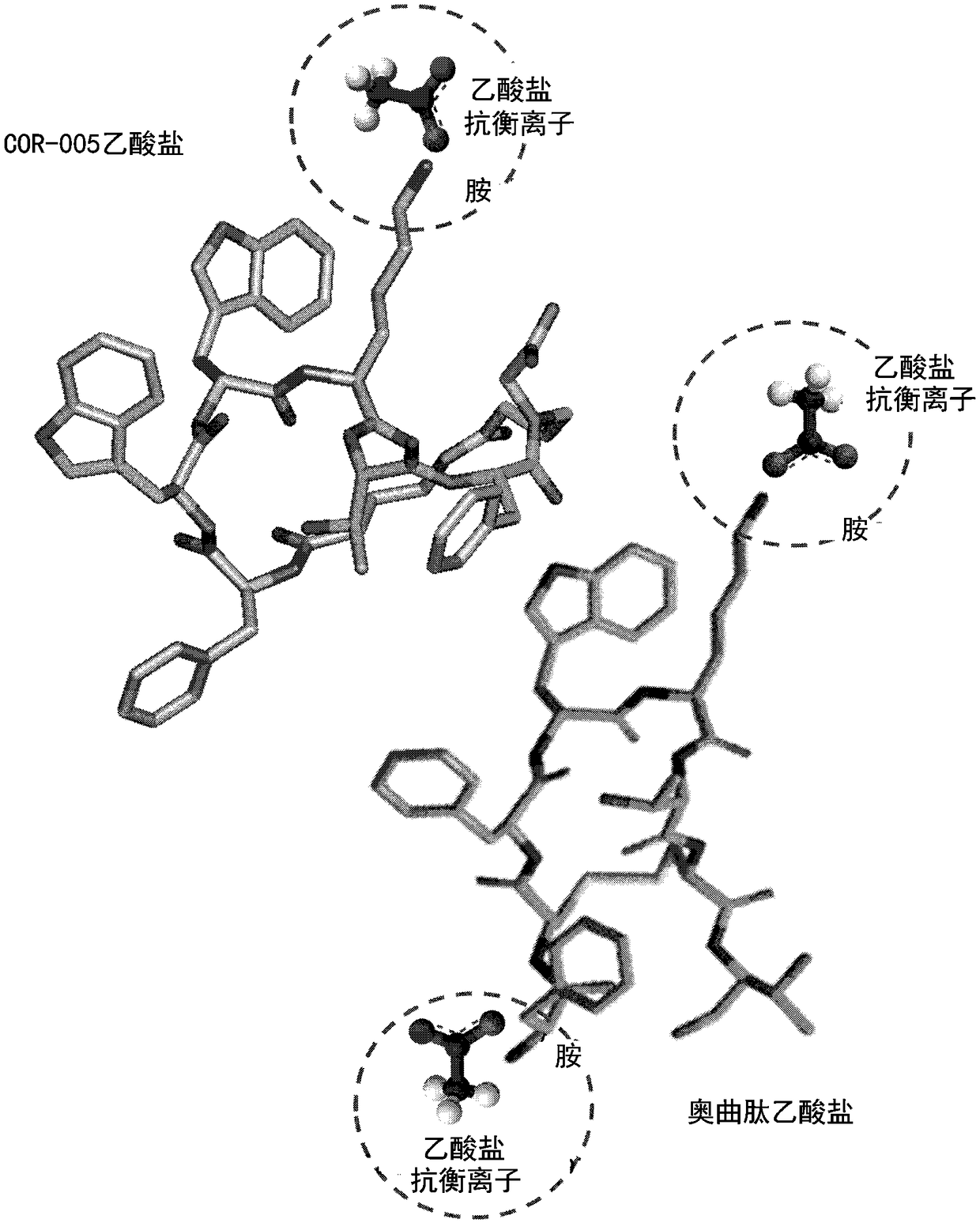
Veldoreotide with poor solubitliy in physiological conditions for use in the treatment of acromegaly, acromegaly cancer, sst-r5 expressing tumors, type 2 diabetes, hyperglycemia, and hormone-related tumors
A technology of vedoretide and dextrose, applying vedoretide with poor solubility under physiological conditions for the treatment of acromegaly, acromegaly cancer, SST-R5 expressing tumor, type II In the field of diabetes, hyperglycemia and hormone-related tumors, it can solve the problems of poor solubility, increased dosage, low solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] The solubility of vedoretide acetate in aqueous media was screened by the following method. A fixed amount of 10 mg of peptide was weighed and dissolved by increasing volume (incremental) of the test medium at ambient temperature until complete solubility was observed, i.e. a clear / transparent solution and no precipitation occurred (solubility was determined according to USP guidelines endpoint value). Following the results of the screening studies, various media were selected for validation studies based on quantitative assessment of peptide concentration (maximum solubility) by HPLC. The results of the screening study are described in Table 1, while the results of the validation study are included in Table 2. From the data it can be concluded that vedoretide acetate exhibits high solubility in water and other aqueous media, while being limited by limited solubility in isotonic media.
[0162] Table 1: Solubility Screening of Vedoretide Acetate
[0163]
[0164] ...
Embodiment 2
[0187] The effect of vedoreotide acetate on the density, pH and surface tension of water is shown in Table 4. It has been found that vedoreotide acetate behaves as a peptide amphiphile and lowers the surface tension of water. However, the reduction in water surface tension was not in a dose-dependent manner, suggesting that vedoretide acetate acts as a hydrotrope rather than a surfactant.
[0188] Tween 80 was used as a control surfactant for comparison. Density was measured with a fixed volume of 1 mL of sample. All tests were repeated three times at 25°C. The effect of vetoreotide acetate on the pH of double distilled water (DDW) was the same at all vetoreotide acetate concentrations. The pH 5.0 of DDW was lowered to pH 4.5. The effect of vedoretide acetate on DDW density (the initial density of DDW is 1.003g / ml) is concentration-dependent from 1.4mg / ml to 21.5mg / ml, and the density is in the range of 0.986 to 0.998g / ml respectively . However, at vedoretide acetate con...
Embodiment 3
[0222] Preparation of Vedoretide Acetate Immediate Release Formulations (Groups 1M-6M) for Injection into HSD: SPRAGUE In rats, as shown in Table 8 below. The lactic acid vehicle for the formulations shown in Table 7 below was prepared as follows:
[0223] Table 7
[0224]
[0225] The formulations prepared for rat injection are summarized in Table 8.
[0226] Each treatment was injected into test rats in groups of 5 rats (average weight of each subject was about 370 g) according to the above-mentioned dosing regimen. Baseline tail blood samples from rats were collected 2 days prior to dosing. For group 1M, blood samples were taken at 5 minutes, 10 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours and 24 hours after dosing. For groups 2M-6M, tail blood samples were collected at 30 minutes, 1 hour, 2 hours, 3 hours, 6 hours, 8 hours, 12 hours and 24 hours after dosing. Blood samples were approximately 300 uL whole blood collected in commercially available...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com