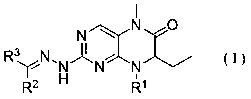
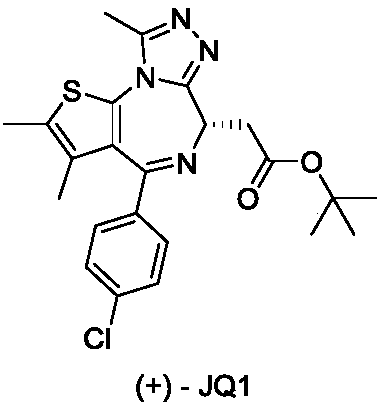
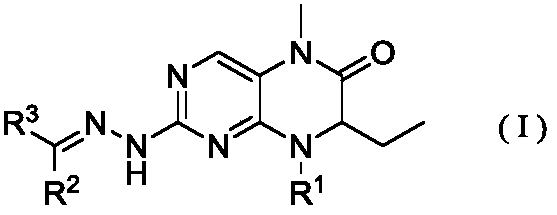
Dihydropteridine ketone type BRD4 (Bromodomain-Containing Protein 4) protein inhibitor as well as preparation method and application thereof
A technology of dihydropteridone and dihydropteridine, which is applied in the fields of organic chemistry, drug combination, antitumor drugs, etc., can solve the problem that there are not many BRD4 small molecule inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Preparation of 2-aminobutyric acid methyl ester hydrochloride:
[0100] Add 2-aminobutyric acid (1.00g, 1.0eq.) into a 50mL eggplant-shaped flask, add 15mL of anhydrous methanol, and slowly add SOCl at 0°C 2 (2.31g, 2.0eq.), after heating to reflux for 1.5 hours, TLC showed that the reaction was complete. Evaporation of the volatiles gave a white solid which was analyzed with Et 2 O ground the solid, obtained the crude product of compound 2 after suction filtration, and could directly carry out the next step reaction without purification, and the yield was 90%.
[0101] White solid, melting point: 120-122℃. 1 H NMR (400MHz,D 2 O)δ4.04(t,J=6.2Hz,1H),3.76(s,3H),1.99–1.82(m,2H),0.97–0.87(m,3H). 13 C NMR (101MHz,D 2 O) δ170.79, 53.99, 53.46, 23.21, 8.42. HR-MS (ESI): Calcd.C 5 h 12 ClNO 2 ,[M+H] + m / z:118.0863,found:118.0867.
Embodiment 2
[0103] The preparation of 2-(cyclopentylamino) methyl butyrate:
[0104] Compound 2 (1.00g, 1.0eq.) and cyclopentanone (548mg, 1.0eq.) were ice-bathed for 10 minutes, and NaOAc (547mg, 1.0eq.) and NaBH(OAc) were added 3 (2.07g, 1.5eq.). The reaction mixture was stirred overnight at room temperature. After TLC showed that the reaction was over, add saturated NaHCO to the reaction system 3 solution to quench the reaction, then CH 2 Cl 2 (20mL×2) extract the aqueous layer, combine the organic layers, wash over anhydrous Na 2 SO 4 Drying, filtration and concentration afforded compound 3 in 65% yield.
[0105] Colorless oily liquid. 1 H NMR (400MHz, DMSO-d 6 )δ3.63(s,3H),3.10(t,J=6.6Hz,1H),2.98–
[0106] 2.84(m,1H),1.80(s,1H),1.67–1.54(m,4H),1.54–1.48(m,2H),1.47–1.39(m,2H),1.32–1.21(m,2H), 0.84(t,J=7.4Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ175.71, 60.88, 57.32, 51.19, 33.17, 32.10, 26.25, 23.38, 23.33, 10.21. HR-MS (ESI): Calcd.C 10 h 19 NO 2 ,[M+H] + m / z:186.1494,fou...
Embodiment 3
[0108] Preparation of 2-((2-chloro-5-nitropyrimidin-4-yl)(cyclopentyl)amino)butanoic acid methyl ester:
[0109] Compound 3 (1.00g, 1.0eq.) was added to 15mL of acetone, and after 10 minutes of ice bathing, 2,4-dichloro-5-nitropyrimidine (1.05g, 1.0eq.) was added, and K 2 CO 3 (750mg, 1.0eq.), stirred overnight at room temperature. After the reaction was complete, the solvent was removed, the residue was dissolved with ethyl acetate, and washed with water, the organic layer was collected, dried over anhydrous sodium sulfate, and purified by silica gel flash column chromatography (PE / EA=10:1) to obtain compound 4, yielding The rate is 89%.
[0110] Yellow solid, melting point: 110-111℃. 1 H NMR (400MHz, DMSO-d 6 )δ8.84(s,1H),4.29–4.14(m,1H),3.64(s,3H),3.52–3.48(m,1H),2.28–2.15(m,1H),2.04–1.90(m, 2H),1.85–1.77(m,2H),1.72–1.61(m,3H),1.51–1.35(m,2H),0.94(t,J=7.8Hz,3H). 13 C NMR (101MHz, DMSO-d 6 )δ164.21, 151.73, 151.14, 137.83, 119.27, 61.06, 59.38, 28.30, 28.28, 26.36, 23...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com