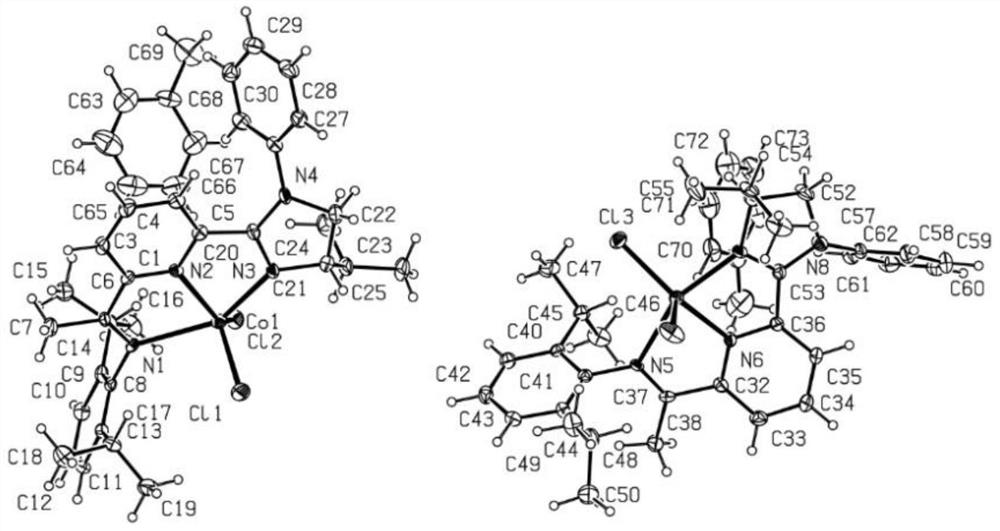
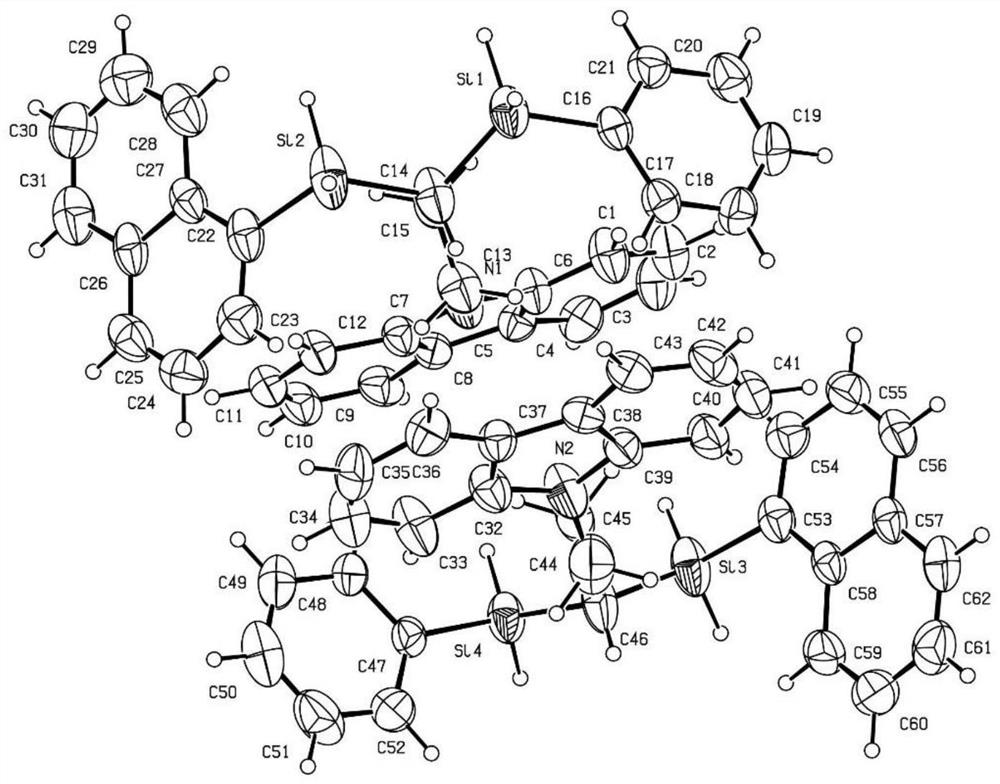
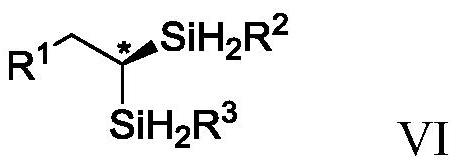A chiral gem-disilazane compound containing four silicon-hydrogen bonds and its synthesis method and application
A technology of disilazane and compound, applied in the field of chiral gem disilazane compound containing four silicon-hydrogen bonds and its synthesis and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Chiral CoX 2 Asymmetric Hydrosilylation of Alkenyl Silicon and Silane Catalyzed by -IIP Complex
[0087] Add chiral CoX sequentially to a dry reaction tube at room temperature under argon 2 -IIP complex (0.015mol, 3mol%), toluene (2.0mL), trihydrosilane (0.55mmol) shown in formula IV, sodium triethylborohydride (0.045mol, 9mol%), shown in formula V alkenyl silicon (0.5 mmol). After stirring at room temperature for 2 hours, the product was obtained by column chromatography (elution solvent was petroleum ether or a mixture of petroleum ether and ethyl acetate). (in individual reaction conditions, chiral CoX 2 The amounts of -IIP complex and sodium triethylborohydride varied and are specified under each product. )
[0088]
[0089] VI-1:
[0090] (R)-Benzyl(1-(phenylsilyl)hexyl)silane
[0091] (R)-benzyl(1-(phenylsilyl)hexyl)silane
[0092]
[0093] Oily liquid, 83% yield, regioselectivity >19 / 1, [α] 20 D =-8.3 (c 1.02, CHCl 3 ),98.8%ee, IR(cm ...
Embodiment 2
[0128] Example 2: Chiral CoX 2 Asymmetric hydrosilylation of alkynes and two different trihydrosilanes catalyzed by -IIP complex
[0129] Add Co(OAc) sequentially to a dry reaction tube at room temperature under argon 2 (0.01mmol), Dpephoes (0.012mmol), toluene (2.0mL), trihydrosilane (0.50mmol) shown in formula II, sodium triethylborohydride (0.03mol), alkyne (0.50mmol) shown in formula I mmol), stirred at room temperature for 3 hours. Then, under the protection of argon, add CoX 2 -IIP (0.015mol), trihydrosilane (0.50mmol) shown in formula VI, sodium triethylborohydride (0.045mol) was stirred at room temperature for 3 hours and separated by column chromatography (elution solvent is sherwood oil or sherwood oil and ethyl acetate) to give the product.
[0130]
[0131] In Example 2, chiral CoX 2 The chemical formula of the -IIP complex is shown in the following formula III-1:
[0132]
[0133] The preparation method is the same as described in Implementation 1.
[0...
Embodiment 3
[0156] Embodiment 3: product oxidation synthesis aldehyde compound (application example)
[0157]
[0158] In a 20mL reaction tube, add VI (1.0mmol), chloroform (5mL), HBF 4 .OEt 2 (6mmol, 40%Wt), the mixture was refluxed for 24 hours, the solvent was spun off, and then tetrahydrofuran (1mL), methanol (3mL), tetrabutylammonium fluoride (8.0mmol), potassium bicarbonate (8.0mmol) were added in sequence ,H 2 o 2 (50mmol, 30%wt).Stir at room temperature for 10h, saturated NaHSO 3 The solution was diluted, extracted three times with ether, washed with saturated brine, dried over anhydrous sodium sulfate, spin-dried, and passed through a column with PE / EtOAc=20 / 1 to obtain compound A (reference for experimental procedures: Chem.Commun.2002, 114-115. )
[0159] The following products were oxidized into aldehyde compounds with reference to the above method, as shown in Table 1.
[0160] Table 1 Oxidative synthesis of aldehydes from chiral gem-disilazane products
[0161] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com