2-amino-3-hydroxypyridine and its preparation method and purification method
A technology of hydroxypyridine and hydroxypyridine sulfonate, which is applied in the direction of organic chemistry, can solve the problems of high raw material prices and low yields, and achieve the effects of high prices, avoiding side reactions, and promoting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
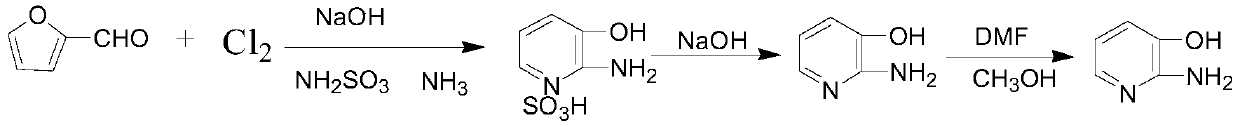
[0041]A preparation method of brown 2-amino-3-hydroxypyridine, which comprises:
[0042] Add 350g of water into the first reaction vessel, add 20g of furfural, stir and cool down to 0°C, and start to introduce chlorine gas to carry out the ring-opening reaction. The conditions of the ring-opening reaction include: introducing chlorine gas under continuous stirring, while keeping the reaction temperature at 0-10°C, and the rate of introducing chlorine gas at 2.5-3g / 10min, wherein furfural is added every 20 minutes after the chlorine gas is passed. , add three times altogether, add 10g each time, during, feed chlorine 37g altogether.
[0043] Transfer the mixed liquid agent obtained after the ring-opening reaction to the second reaction container, drop the liquid alkali after cooling to 0°C, adjust the pH of the mixed liquid agent to keep at 1.5-2, and keep the pH of the mixed liquid agent at 1.5-2 The temperature of the mixed solution is 0-10°C, and the time for dropping is ab...
Embodiment 2
[0050] A white 2-amino-3-hydroxypyridine obtained by refining the brown 2-amino-3-hydroxypyridine provided in Example 1 by the following refining method, the refining method comprising:
[0051] Add 45g of brown 2-amino-3-hydroxypyridine obtained in Example 1 into the fourth reaction vessel, add 200ml of dimethylformamide, heat up to 100°C±2°C for 30min, filter while it is hot, and add the filtrate to the fifth In the reaction vessel, the temperature was rapidly cooled to -5°C using an ice-salt bath, and filtered to obtain a white solid.
[0052] Add all the white solid to the sixth reaction vessel, add 200ml of methanol to raise the temperature and reflux for 1 hour, cool down to -5°C, filter, and dry to obtain dry white 2-amino-3-hydroxypyridine.
[0053] Among them, 40g of dry white 2-amino-3-hydroxypyridine has a melting point of 172-174°C. As determined by high performance liquid chromatography, the purity of white 2-amino-3-hydroxypyridine is 99.9%. Calculated, the yiel...
Embodiment 3
[0055] A kind of preparation method of brown 2-amino-3 hydroxypyridine, it comprises:
[0056] Add 370g of water into the first reaction vessel, add 19g of furfural, stir and cool down to 0°C, and start to introduce chlorine gas to carry out the ring-opening reaction. The conditions of the ring-opening reaction include: feeding chlorine gas under continuous stirring, while keeping the reaction temperature at 0-8°C, and the feeding rate of chlorine gas is 2.6-3g / 10min, wherein furfural is added every 20 minutes after chlorine gas feeding , add four times altogether, add 8g each time, during, feed chlorine 39g altogether.
[0057] Transfer the mixed liquid agent obtained after the ring-opening reaction to the second reaction container, drop the liquid alkali after cooling to 0°C, adjust the pH of the mixed liquid agent to keep at 1.5-2, and keep the pH of the mixed liquid agent at 1.5-2 The temperature of the mixed solution is 0-8°C, and the time for dropping is about 2 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com