Direct antiglobulin testing capability verifying method and testing kit thereof
A technology for proficiency verification and detection capabilities, applied in biological testing, material inspection products, measuring devices, etc., can solve problems that cannot be used to evaluate direct resistance detection capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
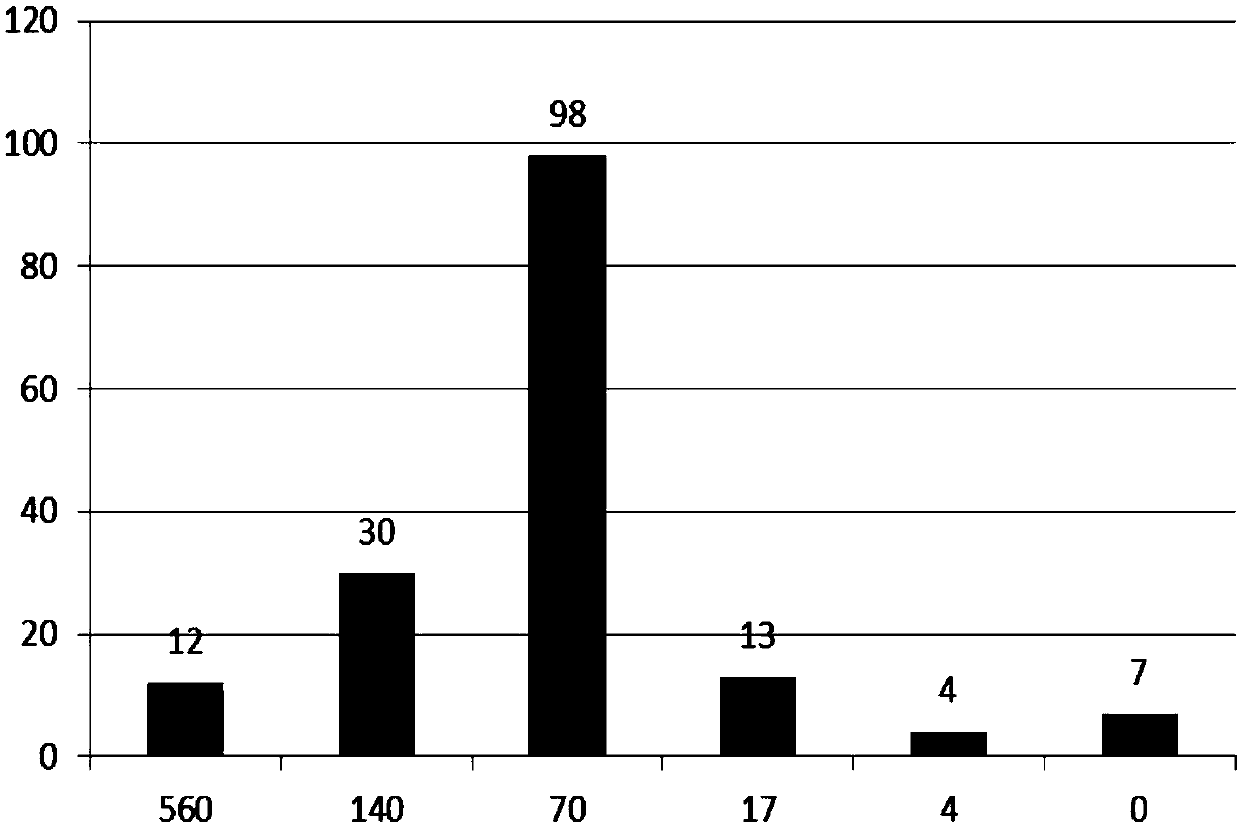
[0034] 1. Preparation of different direct resistance positive proficiency test products: use direct resistance negative, fresh, multi-person mixed red blood cells with a certain concentration, add a series of IgG blood type antibodies with a certain concentration, and prepare 3 to 12 different direct resistance strengths of test red blood cells. Since the concentration of red blood cells is known, the amount of IgG antibody added is known. Through calculation, the average number of IgG antibodies on each red blood cell can be obtained in direct anti-positive samples with different intensities. Through the proficiency testing product composed of a series of test products with known direct anti-positive strength and several direct anti-negative "interference items", the relative quantitative evaluation of the ability of the participating laboratories to detect direct anti-positive can be carried out, and can be performed in The test results of different times are compared to fin...
Embodiment 2
[0042] see Figure 7 , Figure 7 It is a flow chart of the direct resistance detection ability verification method of the present invention. Include the following steps:
[0043] 1. First, prepare different direct-resistance positive proficiency test products or mixed agglutination direct-resistance positive proficiency test products.
[0044] The preparation method of the different direct-antibody-positive proficiency test products is as follows: use direct-antibody-negative, fresh, mixed red blood cells with a certain concentration, add a series of IgG blood-type antibodies with a certain concentration, and prepare 3 to 12 different direct-antibody antibodies. Intensity of test red blood cells, according to the concentration of red blood cells and the amount of IgG antibody added, determine the average number of IgG antibodies on a single red blood cell in each blood group antibody, the above-mentioned series of test red blood cells with different direct positive strengths...
Embodiment 3
[0050] A proficiency testing kit for direct resistance detection, comprising: (1) detection proficiency testing products, including different direct resistance positive proficiency testing products or mixed agglutination direct resistance positive proficiency testing products; the different direct resistance positive proficiency testing products are a group It consists of red blood cell test samples with different direct resistance positive strengths and direct resistance negative red blood cell test samples. The direct resistance positive strength refers to the number of IgG antibodies on a single red blood cell in the sample, and the number of IgG antibodies on a single red blood cell ranges from 4 to 560 The mixed agglutination direct anti-positive proficiency verification product is a group of red blood cell test samples mixed with direct anti-positive red blood cells and direct anti-negative red blood cells in different proportions, and the ratio range is 0.2-8%; (2) Instru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com