Fusion proteins of human protein fragments to create orderly multimerized immunoglobulin Fc compositions with enhanced Fc receptor binding
A technology of multimerization and composition, applied in the fields of autoimmunity, inflammation and tumor immunology, and immunology, which can solve problems such as the ability of unclear molecules to bind to FcγR or complement proteins.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
[0220]Oral or sublingual dissolvable tablet: Angina, polyarteritis nodosa.
[0221] Intravenous, intramuscular, or subcutaneous: myasthenia gravis, hemolytic uremic syndrome (HUS), atypical hemolytic uremic syndrome (aHUS), paroxysmal nocturnal hemoglobinuria (PNH), membranous nephropathy, Neuromyelitis optica, antibody-mediated allograft rejection, lupus nephritis, membranous proliferative glomerulonephritis (MPGN), idiopathic thrombocytopenic purpura, inclusion body myositis, paraproteinaemic IgM decapitation Myelinating polyneuropathy, necrotizing fasciitis, pemphigus, gangrene, dermatomyositis, granuloma, lymphoma, sepsis, aplastic anemia, multisystem organ failure, multiple myeloma of undetermined significance, and Clonal gammopathy, chronic inflammatory demyelinating polyradiculoneuropathy, inflammatory myopathy, thrombotic thrombocytopenic purpura, myositis, anemia, neoplasia, hemolytic anemia, encephalitis, myelitis, spinal cord disease (particularly associated with h...
example 1
[0264] Example 1: Common Storado body
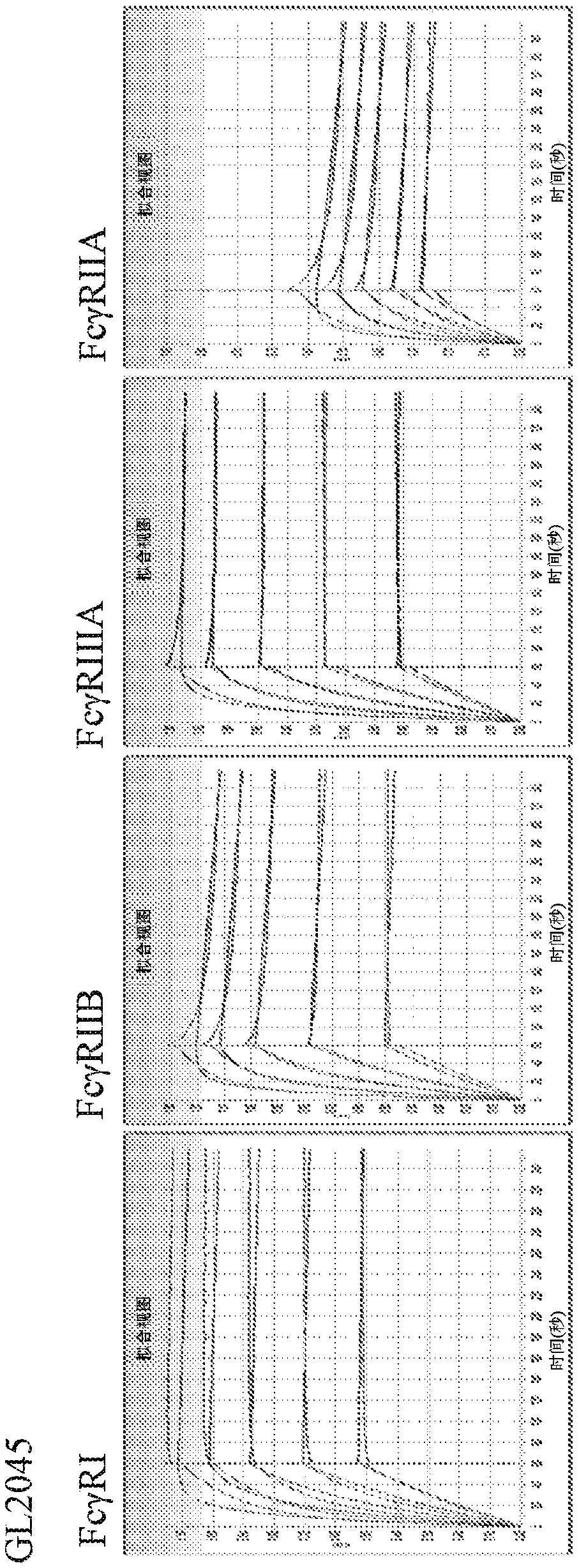
[0265] Various methods were employed to generate Storaolibodies with enhanced classical binding and enhanced complement fixation. Trimers were generated in which at least one point mutation was introduced into the Fc domain. Specifically, the 233rd, 234th, 235th, 236th, 267th, 268th, 299th positions of the Fc domain of the GL-2045 Storado body described in WO2012 / 016073 , 324th, 345th, 430th and 440th positions were mutated. The amino acid sequences of exemplary Stora polysomes are shown in Table 1 above.
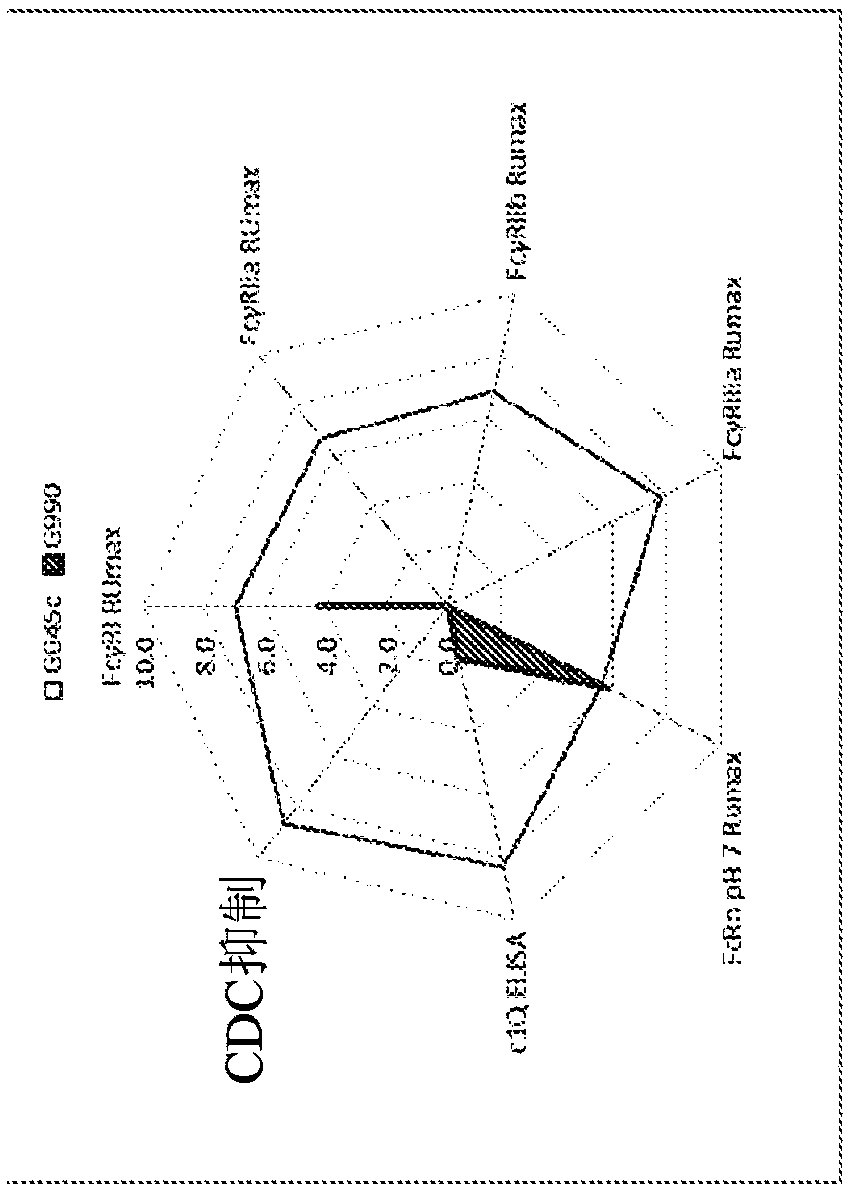
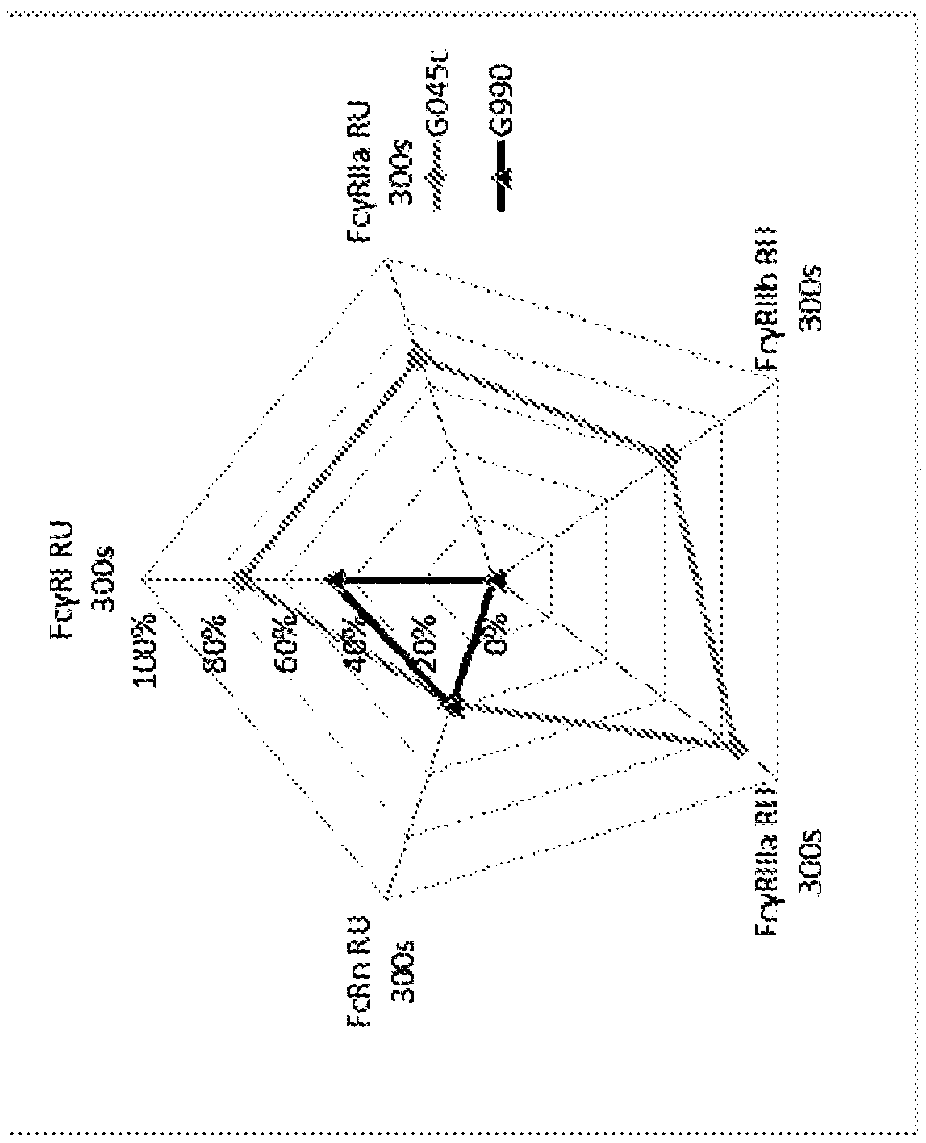
[0266] For each Storado body generated, the levels of canonical FcγR binding, complement C1q binding, and CDC inhibition were determined and compared to the parental Storado body, GL-2045 (IgG1 hinge-IgG1CH2IgG1CH3-IgG2 hinge).
[0267]Binding of the common Storado or the parental Storado, GL-2045, to FcyRI, FcyRIIb, FcyRIIIa, FcyRIIa was assessed. Dissociated RU values were measured by biolayer interferometry using a ForteBio...
example 2
[0276] Example 2 - Enhanced Complement Fixation by Common Storadobody
[0277] A study was performed to evaluate the binding of common Storados to C1q, the results of which are summarized in Table 3.
[0278] For C1q binding, 96-well plates were coated overnight with C1q (Sigma Cat#: C1740 1 μg / mL) in PBS. After coating, plates were washed 3 times with standard wash buffer (PBS + 0.05% Tween 20) and blocked with blocking buffer (1% BSA-0.05% PBS Tween) for 2 hours at room temperature. After blocking, plates were incubated with 100 μL / well of compound diluted in blocking buffer and washed 3 times with standard wash buffer. By incubating with 1:5000 biotinylated mouse anti-human IgG1 (Cat#555869, BD Biosciences) and streptavidin-HRP (Cat#:7100-05Southern Biotech) (100 μL / well) at room temperature After 1 hour, followed by 3 washes with wash buffer, C1q-bound compounds were developed for 15 minutes using the standard TMB method according to the manufacturer's protocol. Absor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com