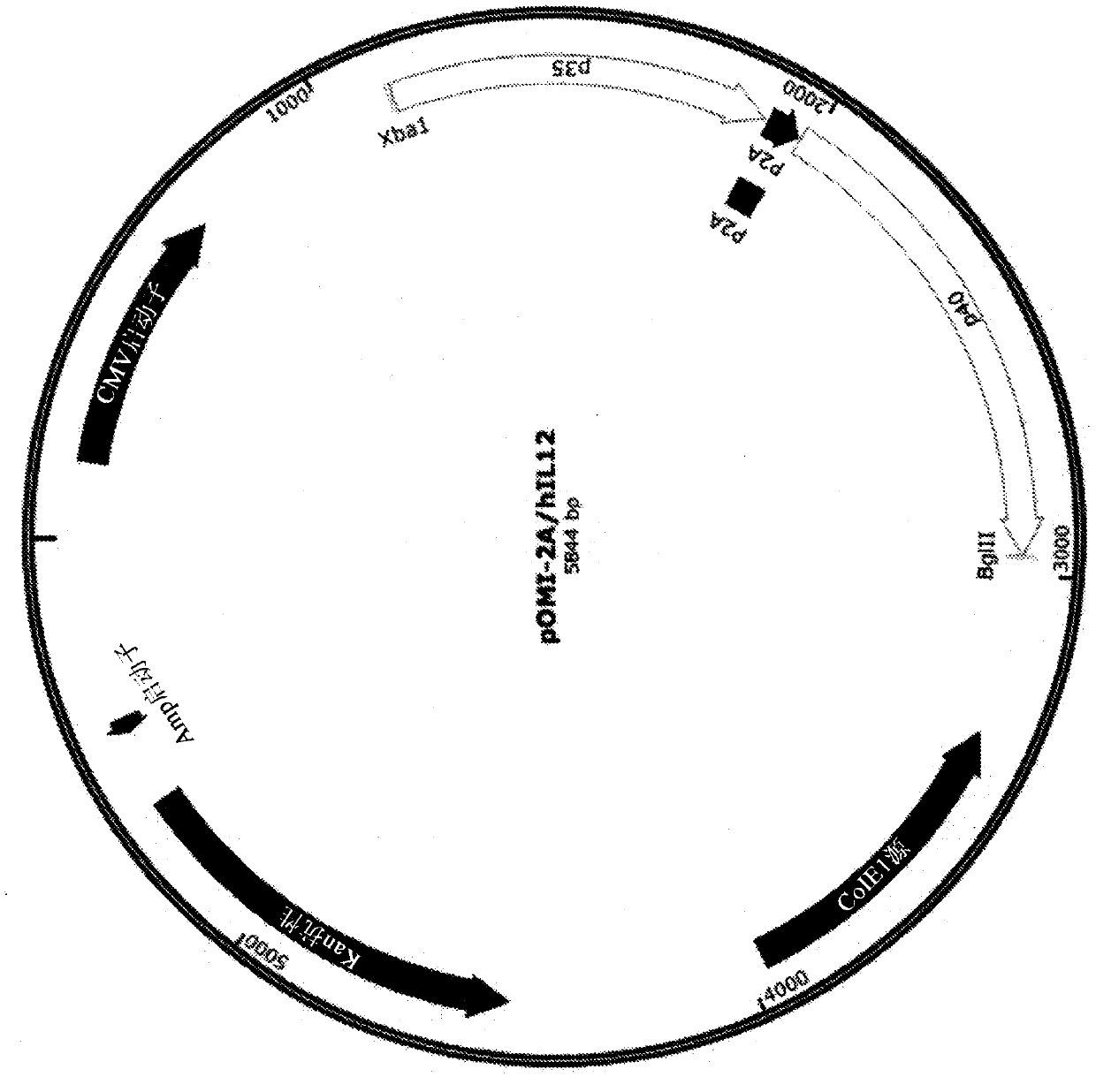
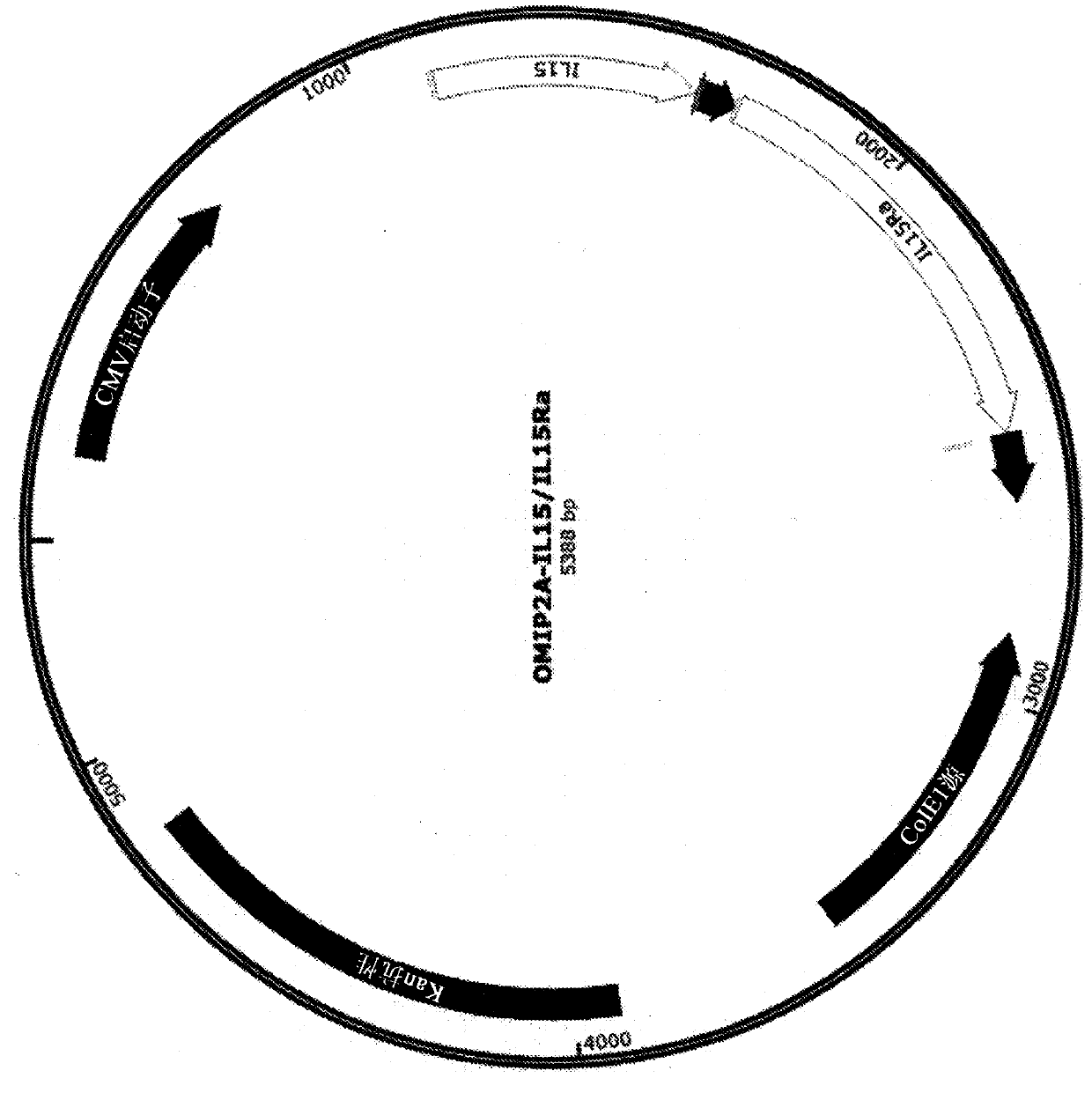
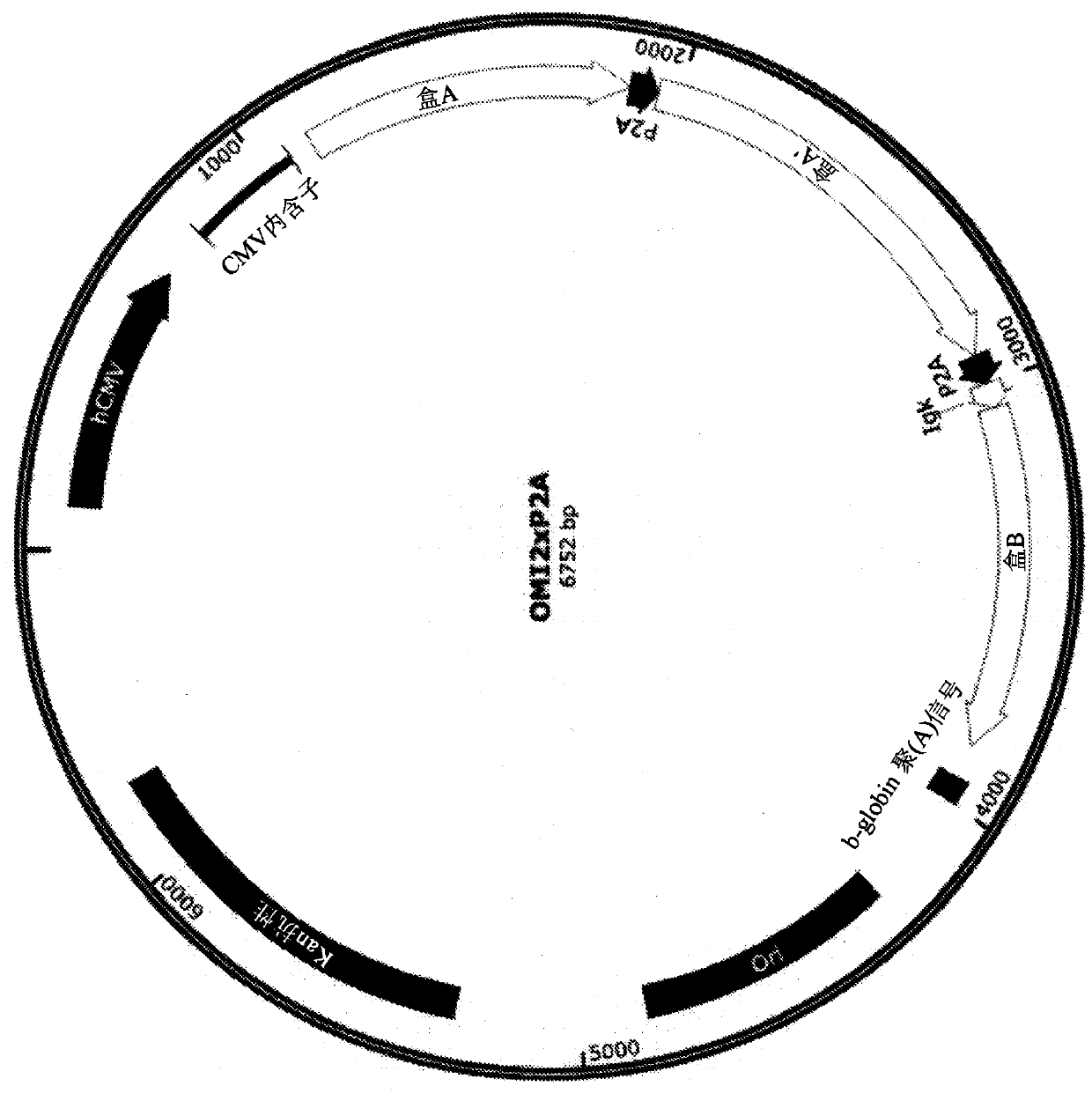
Plasmid constructs for heterologous protein expression and methods of use
A technology for expressing plasmids and structures, applied in the fields of peptide/protein components, chemical instruments and methods, animal/human proteins, etc., can solve the problems that cannot meet the needs of sufficient production of immune regulatory proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0101] I. General method
[0102] Describes standard methods in molecular biology. Maniatis et al. (1982) "Molecular Cloning: A Laboratory Manual", Cold Spring Harbor Laboratory Press, New York; Sambrook and Russell (2001) "Molecular Cloning", 3rd edition , Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York; Wu (1993) "Recombinant DNA", Volume 217, San Diego Academic Press, California. Standard methods are also presented in Ausbel et al. (2001) "Current Protocols in Molecular Biology", Volumes 1 to 4, John Wiley Publishing Co., Ltd., New York, USA, described in "Molecular Biology Latest Protocols" Bacterial cells and DNA mutation clones (Volume 1), mammalian cells and yeast clones (Volume 2), glycoconjugates and protein expression (Volume 3) and Bioinformatics (Volume 4).
[0103] Describes protein purification methods including immunoprecipitation, chromatography, electrophoresis, centrifugation, and crystallization. Coligan et al. (2000) "Current Protocols in Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com