Assay for determining potential to self-association of a protein using concentration-dependent self-interaction nanoparticle spectroscopy
A technology of nanoparticles and gold nanoparticles, which can be used in measurement devices, biological testing, and analysis by causing chemical reactions to occur in materials, which can solve problems such as instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0193] Example 1: Materials
[0194] Monoclonal antibody (mAb) by Cells are generated and purified by protein A chromatography and the polishing step of anion exchange or hydrophobic interaction chromatography. The buffer components are obtained from Sigma-Aldrich, VWR or JT-Baker and are the highest grade available. Illustra NAP column (Cat. #17-0853-02) and XK26 / 100 Superdex200pg column (Cat. #90-1002-73) were purchased from GE Healthcare Life Sciences. Amicon centrifugal filtration device (Cat. #UFC905024) was purchased from EMD Millipore. Slide-A-LyzerTM G2 dialysis cassette (Cat. #87732) was purchased from ThermoFisher Scientific. 20nm gold nanoparticles (Cat.#HD.GC20) were purchased from BBI solutions. ThermoScientificTM NuncTM MicrowellTM 96-well microplate (Cat.#12-565-66) was used as a reaction vessel to obtain absorption spectra.
example 2
[0195] Example 2: High-concentration static light scattering method (HC-SLS).
[0196] In 10mM MES, pH 6.0, 50mM sodium chloride, through XK26 / 100Superdex 200pg column Each concentrated (100 g / L) monoclonal antibody (mAb1, mAb2, mAb3, mAb4, mAb5 and mAb6) was purified on avant (GE Healthcare Life Sciences). The monomer mAb fraction was collected and concentrated using a series-connected Easy-LoadMAsterFlexL / S (Cole Parmer) pump and VivaFlow 2030,000 MWCO HY membrane. Divide 150 mL of each concentrated antibody into 3 parts, and pack them into 10,000 MWCO dialysis cassettes, and follow 2 L of 10 mM MES, pH 6.0, 250 mM sodium chloride; 10 mM MES, pH 6.0, 50 mM sodium chloride; and 10 mM MES , PH 6.0, 10mM sodium chloride solution exchange. A centrifugal filter unit with 50,000 MWCO was used to concentrate the solution to a final volume of approximately 15 mL in the corresponding buffer. The concentration was measured using SoloVPE (CTechnologies, Inc.). In 10mM MES, pH 6.0, an...
example 3
[0197] Example 3: Standard Self-Interacting Nanoparticle Spectroscopy (SINS).
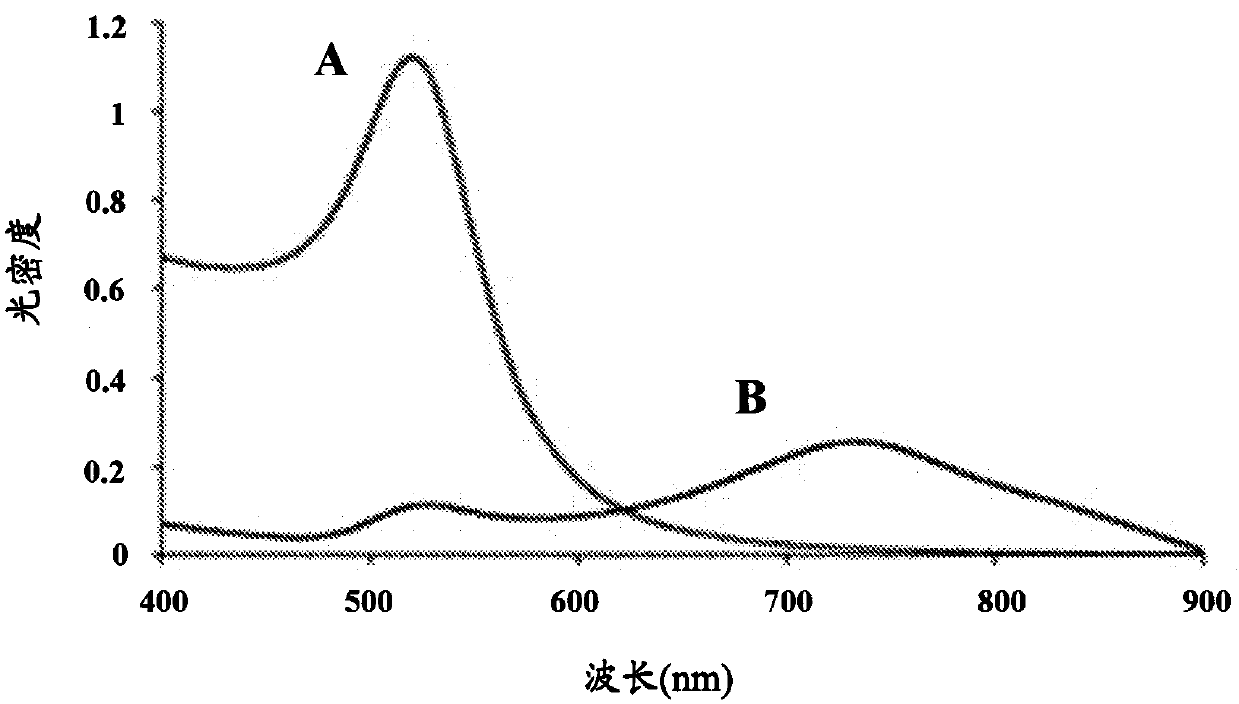
[0198] Sub-aliquots of each antibody (mAb1, mAb2, mAb3, mAb4, mAb5, and mAb6) under different salt conditions were added to separate 15 mL centrifuge tubes. 5mL of 1 optical density (1O.D) 20nm gold nanoparticle solution was added to the solution, so that the final protein concentration obtained was 50μg / mL. After waiting for 30 minutes, the absorption spectrum and λ max Recorded in SPECTRAmax 340PC (Molecular Devices).
[0199] Concentration-dependent self-interacting nanoparticle spectroscopy (CD-SINS). Prepare a 100 mM buffer solution at a suitable pH. Adjust the Illustra NAP column with 2.4 mL of 100 mM buffer solution (for example, MES or sodium phosphate, depending on the target pH). Use the adjusted desalting column to buffer exchange the concentrated (50-75 g / L) mAb stock solution. Use SoloVPE to measure the result Concentration. Then each antibody was diluted to 5.12mg / mL in 100mM buffer. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com