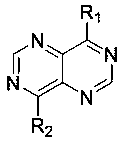
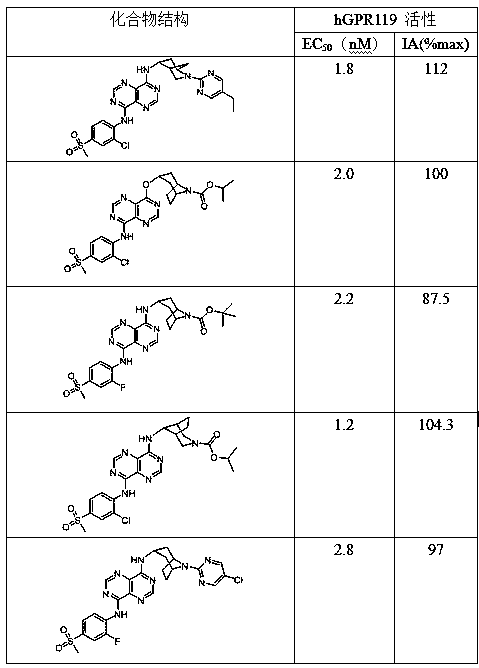
Pyrimidopyrimidine type derivative as well as preparation method and application thereof to medicines
A technology of pyrimidine and derivatives, applied in the field of chemical drugs, can solve problems such as fluid retention, weight gain, and decreased β-cell function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 2,6,8-Trichloro-N-(4-thiamphenyl)phenyl)pyrimido[5,4- d ]pyrimidin-4-amine;
[0030] Add tetrachloropyrimido[5,4- d ]pyrimidine (0.65 g, 2.4 mmol), THF (5 mL) and H 2 O (0.6 mL). 4-Methylthioaniline (0.1 mL, 0.8 mmol) was added at 0 °C and the reaction was stirred at room temperature for 1 h. Join CH 2 Cl 2 (10 mL), mCPBA (0.36 g, 77%, 1.6 mmol) was added at 0°C, and the reaction was stirred overnight at room temperature. CH 2 Cl 2 Diluted, the organic phase was washed with saturated sodium thiosulfate solution, saturated sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation, and the crude product was subjected to silica gel column chromatography (petroleum ether: ethyl acetate=5: 1) Purification gave a yellow solid, and the yield of the two-step reaction was 32%. 1 H-NMR (600 MHz, CDCl 3 ) δ (ppm): 9.12 (s, 1H), 8.20-8.00 (m, 4H), 3.10 (s, 3H).
Embodiment 2
[0032] 2,6,8-Trichloro-N-(2-fluoro-4-thiamphenicol)phenyl)pyrimido[5,4- d ]pyrimidin-4-amine;
[0033] Referring to the preparation method of Example 1. 1 H-NMR (600 MHz, CDCl 3 ) δ (ppm): 9.37 (s, 1H), 8.99(t, J = 8.5 Hz, 1H), 7.95-7.80 (m, 2H), 3.13 (s, 3H).
Embodiment 3
[0035] 2,6,8-Trichloro-N-(2-fluoro-4-cyano)phenyl)pyrimido[5,4- d ]pyrimidin-4-amine;
[0036] Referring to the preparation method of Example 1. 1 H-NMR (600 MHz, CDCl 3 ) δ (ppm): 9.37(s, 1H), 8.94(t, J = 8.3, 1H), 7.66(d, J = 8.6, 1H), 7.58(dd, J = 10.4, 1.7, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com