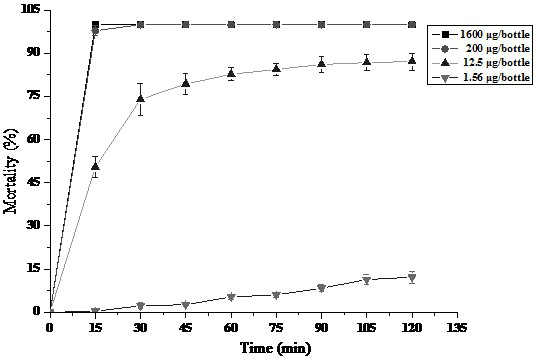
A novel chemical modification of thiomethrin and its preparation method and application
A technology of chemical modification and thiomethrin, applied in chemical instruments and methods, preparation of organic compounds, botany equipment and methods, etc., can solve human and animal health threats, adverse effects on children's health, interference with biological functions, etc. problems, achieve good application prospects, solve the problem of drug resistance, and improve the effect of metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Preparation of Novel Thiolthrin Chemical Modification (LCA-COS-01)
[0037] 1. The preparation of novel thiothrin chemical modification (LCA-COS-01), comprising the following steps:
[0038] (1) First weigh 200mg (0.824mmol) of permethrin in a 50mL round-bottomed flask, and under nitrogen protection, use a syringe to measure 15mL of ultra-dry anhydrous dichloromethane in the flask and stir After it is completely dissolved, add 0.210mL (2.473mmol) of oxalyl chloride three times the amount, stir for about 30min, then add 0.1mL of anhydrous grade N,N-dimethylformamide (DMF) with a 1mL syringe , the reaction will produce a large number of bubbles, and then react at room temperature for 1h;
[0039] (2) The reaction-activated product in the round-bottomed flask after the reaction for 1h is rotary-evaporated and dried in a vacuum rotary evaporator, and during the operation, the external air is reduced as far as possible from entering the round-bottomed flask, an...
Embodiment 2
[0045] Embodiment 2 Preparation of Novel Thiolthrin Chemical Modification (LCA-COS-02)
[0046] 1. The preparation of novel thiothrin chemical modification (LCA-COS-02), comprising the following steps:
[0047] (1) First weigh 200mg (0.824mmol) of permethrin in a 50mL round-bottomed flask, and under nitrogen protection, use a syringe to measure 15mL of ultra-dry anhydrous dichloromethane in the flask and stir After it is completely dissolved, add 0.210mL (2.473mmol) of oxalyl chloride three times the amount, stir for about 30min, then add 0.1mL of anhydrous grade N,N-dimethylformamide (DMF) with a 1mL syringe , the reaction will produce a large number of bubbles, and then react at room temperature for 1 hour;
[0048] (2) The reaction-activated product in the round-bottomed flask after the reaction for 1h is rotary-evaporated and dried in a vacuum rotary evaporator, and during the operation, the outside air is reduced as far as possible from entering the round-bottomed flask,...
Embodiment 3
[0054] Embodiment 3 Preparation of Novel Thiolthrin Chemical Modification (LCA-COS-03)
[0055] 1. The preparation of novel thiothrin chemical modification (LCA-COS-03), comprising the following steps:
[0056] (1) First weigh 200mg (0.824mmol) of permethrin in a 50mL round-bottomed flask, and under nitrogen protection, use a syringe to measure 15mL of ultra-dry anhydrous dichloromethane in the flask and stir After it is completely dissolved, add 0.210mL (2.473mmol) of oxalyl chloride three times the amount, stir for about 30min, then add 0.1mL of anhydrous grade N,N-dimethylformamide (DMF) with a 1mL syringe , the reaction will produce a large number of bubbles, and then react at room temperature for 1 hour;
[0057] (2) The product activated in the previous step in the round-bottomed flask after the reaction for 1 h is dried by rotary evaporation in a vacuum rotary evaporator, and during the operation, reduce the external air from entering the round-bottomed flask as much a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com