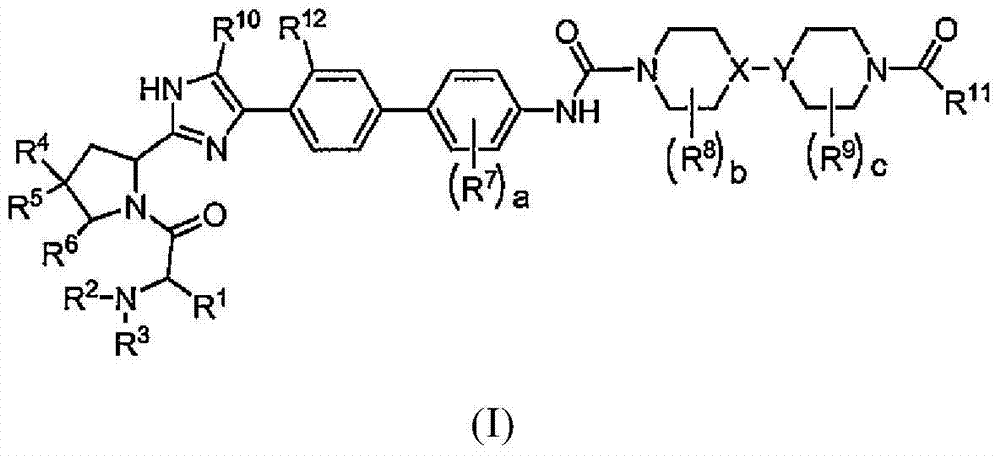
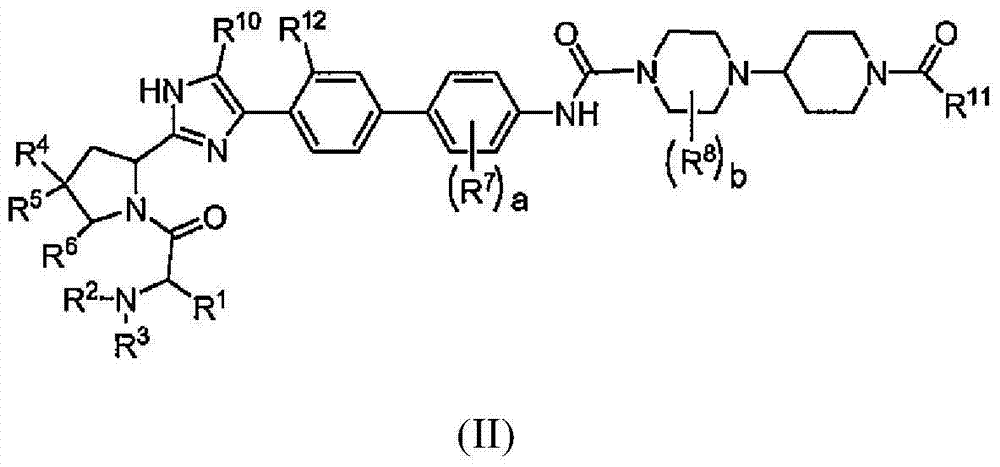
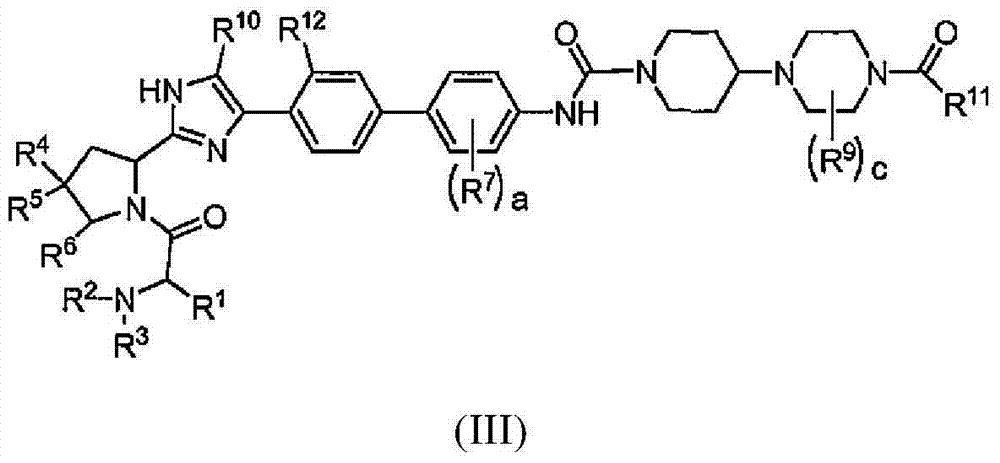
Piperazine-piperidine compounds as hepatitis C virus inhibitors
A technology of compounds and therapeutic agents, applied in the field of piperazine-piperidine compounds as hepatitis C virus inhibitors, capable of solving unmet problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0262] The following synthetic and biological examples are provided to illustrate the invention but are not to be construed in any way as limiting the scope of the invention. In the following examples, the following abbreviations have the following meanings unless otherwise specified. Abbreviations not defined below have their generally accepted meanings.
[0263] ACN = acetonitrile
[0264] AcOH = acetic acid
[0265] CDI = 1,1'-carbonyldiimidazole
[0266] DCM = dichloromethane
[0267] DIPEA = N,N-Diisopropylethylamine
[0268] DMA = N,N-dimethylacetamide
[0269] DMF = N,N-Dimethylformamide
[0270]DMSO = dimethyl sulfoxide
[0271] EDC = N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride
[0272] EtOAc = ethyl acetate
[0273] Et 3 N = Triethylamine
[0274] h = hour
[0275] HATU = N,N,N'N'-tetramethyl-O-(7-azabenzotriazol-1-yl)uronium hexafluorophosphate
[0276] HCTU = 2-(6-chloro-1H-benzotriazol-1-yl)-1,1,3,3-tetramethylammonium hexafluorophosph...
example 1
[0440] Example 1: ((S)-1-{(S)-2-[4-(4'-{[(S)-4-(1-cyclopropanecarbonyl-piperidin-4-yl)-3-methan Base-piperazine-1-carbonyl]-amino}-2'-trifluoromethoxy-biphenyl-4-yl)-1H-imidazol-2-yl]-pyrrolidine-1-carbonyl}-2-methyl methyl-propyl)-carbamate
[0441]
[0442] ((S)-1-{(S)-2-[4-(4'-amino-2'-trifluoromethoxy-biphenyl-4-yl)-1H-imidazole-2-yl) was stirred at RT Base]-pyrrolidine-1-carbonyl}-2-methyl-propyl)-methyl carbamate (20 mg, 0.04 mmol) and p-nitrophenyl chloroformate (8.9 mg, 0.04 mmol) in DMA (5 mL) 30min in the mixture, followed by successive addition of cyclopropyl-[4-((S)-2-methyl-piperazin-1-yl)-piperidin-1-yl]-methanone 2HCl (18mg, 0.06mmol; Preparation 6) and DIPEA (32 μL, 0.18 mmol). The reaction mixture was stirred at RT for 1 h, concentrated, dissolved in 1:1 acetic acid:water (1.5 mL), and purified by reverse phase HPLC to give the di-trifluoroacetate salt of the title product (6.8 mg, 20% yield) . (m / z):C 42 h 53 f 3 N 8 o 6 [M+H] of + The calculated ...
example 2
[0443] Example 2: [(S)-1-((S)-2-{4-[5'-chloro-4'-({4-[1-(2,2-dichlorocyclopropanecarbonyl)-piperidine -4-yl]-piperazine-1-carbonyl}-amino)-2'-trifluoromethoxy-biphenyl-4-yl]-1H-imidazol-2-yl}-4-methyl-pyrrolidine -1-carbonyl)-2-methyl-propyl]-methyl carbamate
[0444]
[0445] 4-[4-(5-Chloro-4'-{2-[(2S,4S)-1-((S)-2-methoxycarbonylamino-3-methyl-butyryl) was stirred at RT -4-Methyl-pyrrolidin-2-yl]-1H-imidazol-4-yl}-2-trifluoromethoxy-biphenyl-4-ylcarbamoyl)-piperazin-1-yl]- A solution of tert-butyl piperidine-1-carboxylate di-trifluoroacetate (12.94 mg, 0.012 mmol; Preparation 14) in 4M HCl in 1,4-dioxane (0.21 mL) for 30 min gave the intermediate {(S)-1-[(2S,4S)-2-(4-(5'-chloro-4'-[(4-piperidin-4-yl-piperazine-1-carbonyl)-amino]- 2'-Trifluoromethoxy-biphenyl-4-yl}-1H-imidazol-2-yl)-4-methyl-pyrrolidine-1-carbonyl]-2-methyl-propyl}-carbamic acid methyl ester.
[0446] A 0.5M solution of 2,2-dichlorocyclopropane-1-carboxylic acid in DMA (27.8 μL, 0.014 mmol) and HATU (5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com