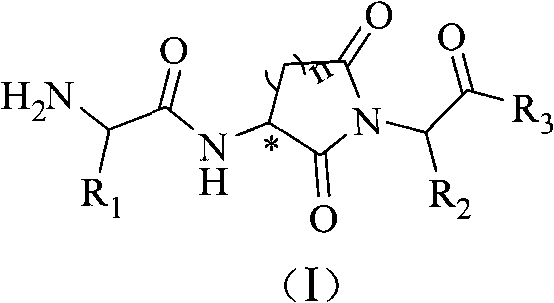
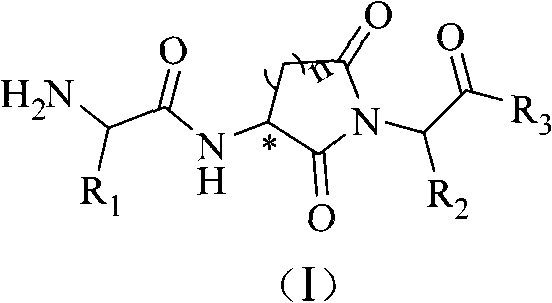
Alpha-amido acyl-ring imide peptoid metalloprotease inhibitor and application thereof
A technology of amino and natural amino acids, which is applied in the field of metalloproteinase inhibitors, can solve the problems of decreased immunity, large number of rotatable single bonds, and reduced T cell recognition ability, etc., to improve compatibility and significantly inhibit enzyme activity in vitro Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
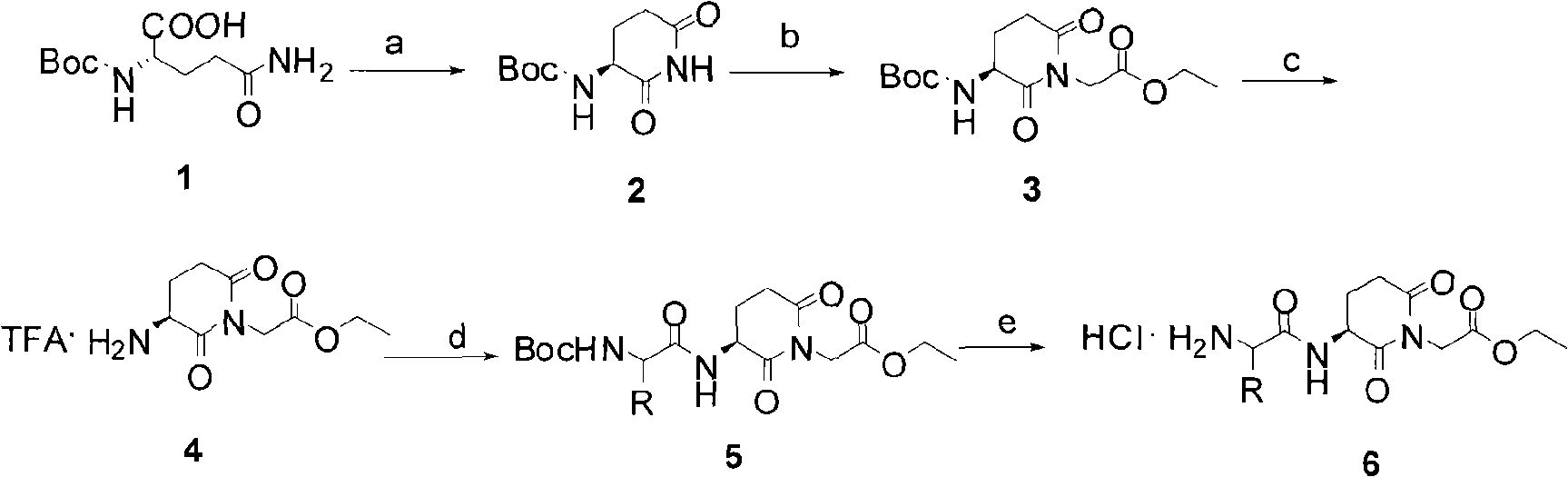
[0116] Example 1. Ethyl 2-((S)-3-((S)-2-amino-3-phenylpropionamido)-2,6-dioxopiperidin-1-yl)acetate salt Preparation of acid salt (6f)
[0117]
[0118] (S)-tert-butyl 2,6-dioxopiperidin-3-ylcarbonamide (2)
[0119] Boc-L-glutamine (41.6g, 0.17mol) and N-hydroxysuccinimide (NIISu, 19.5g, 0.17mol) were dissolved in 200ml THF, and 100ml containing DCC (35.1 g, 0.17mol) in THF. After about 1 hour of dripping, remove the ice and stir in the bathroom for 3 hours, then reflux for 10 hours. The reaction solution was cooled to room temperature, and the solvent was recovered by rotary evaporation. The residue was added to 50 ml of ethyl acetate and concentrated once more. After the final residue was added with 200ml of ethyl acetate, it was frozen in the refrigerator overnight. Filter with 4.0 grams of diatomaceous earth, wash the filtrate with water (50ml*1) and saturated brine (50ml*1), NaSO 4 Dry. Filter and concentrate to dryness. Refined with ethyl acetate-diethyl ether ...
Embodiment 2
[0129] Example 2. 2-((S)-3-((S)-2-Amino-4-methylpentanamido)-2,6-dioxopiperidin-1-yl)acetic acid hydrochloride (11d) preparation of
[0130]
[0131] (S)-Benzyl-2-(3-(tert-butoxycarbonylamido)-2,6-dioxopiperidin-1-yl)acetate (7)
[0132] Compound 2 (18.3g, 0.086mol), K 2 CO 3 (14.2g, 1.2e.q), tetrabutylammonium iodide (TBAI, 3.1g, 0.1e.q.) was swirled in 300ml of anhydrous acetone. Benzyl bromoacetate (30.0 g, 1.5 e.q.) was added slowly at room temperature. The reaction solution was refluxed for 7 hours and cooled to room temperature. Filter and concentrate to obtain a yellow oil. Add 200ml of ethyl acetate, and the organic phase is respectively washed with 0.5% Na 2 CO 3 (20ml*2), 5% citric acid (20ml*2), washed with saturated brine until neutral, dried the organic phase with anhydrous sodium sulfate, filtered, concentrated and recrystallized with ethyl acetate-ether to obtain 20.8g of 7 white crystals , yield 67%. mp 78.0-81.0℃; ESI-MS m / z[M+1] + : 377.6; 1H-NMR...
Embodiment 3
[0140] Example 3. Tert-butyl (S)-1-((S)-1-(2-(hydroxylamino)-2-oxoethyl)-2,6-dioxopiperidine-3-amino)- Preparation of 4-methyl-1-oxopentane-2-carboxylate (13d)
[0141]
[0142] Dissolve 0.2g (3.0mmol) of hydroxylamine hydrochloride in 1.5ml of anhydrous methanol, slowly add 0.5ml (2e.q) of triethylamine to free the amino groups, accurately measure the supernatant, and set aside.
[0143] Dissolve 2.0mmol of carboxylic acid derivative 10d in 20ml of anhydrous THF, control the solution temperature not higher than -20°C, slowly add 4.0mmol (0.57ml) triethylamine, and slowly add 2.02mmol (0.28ml) of chloroformic acid iso Butyl ester (ISC), white turbidity indicates that the reaction to prepare mixed anhydride has occurred. The reaction solution was stirred at -20°C for 15 minutes, and the methanol solution containing hydroxylamine was slowly added dropwise to the mixed anhydride reaction solution, and the drop was completed in about 20 minutes. Continue to keep warm at -20°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com