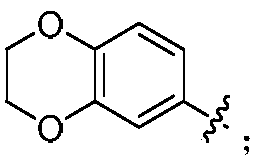
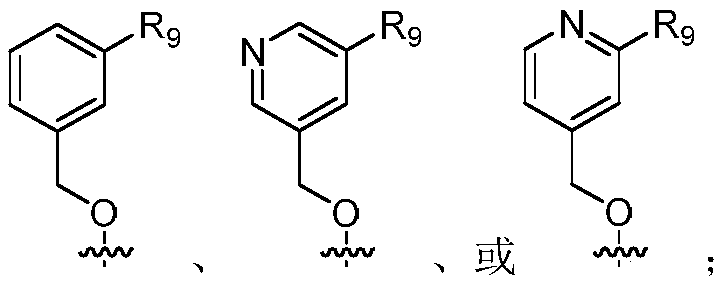
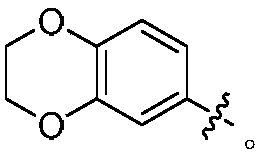
Indoline compounds and preparing method and application thereof
A compound, indoline technology, applied in the field of medicine, can solve the problems of difficult preparation and purification, high production cost, immunogenic toxicity and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: (4-(((2-hydroxyethyl)amino)methyl)phenyl)(4-phenylindolin-1-yl)formyl
[0078] Step 1: 4-Phenyl-1H-indole
[0079] At room temperature, 4-bromo-1H-indole (10g, 51.29mmol), phenylboronic acid (6.26g, 51.29mmol), tetrakistriphenylphosphine palladium (1.78g, 1.54mmol), potassium carbonate (21.23g, 153.94 mmol) was dissolved in a mixed solvent of dioxane and water (volume ratio 4:1, 250 mL). Under the protection of nitrogen, the temperature was raised to 60° C. for 10 hours. Cool to room temperature, suction filter with celite, extract the filtrate with dichloromethane, wash the organic layer with saturated brine, concentrate under reduced pressure, and separate by column chromatography to obtain 6.66 g of white solid with a yield of 67.2%.
[0080] Step 2: 4-Phenylindoline
[0081]
[0082] At room temperature, 4-phenyl-1H-indole (1.5g, 7.77mmol) was dissolved in glacial acetic acid (9mL), and sodium cyanoborohydride (1.47g, 23.32mmol) was added slowly, ...
Embodiment 2
[0091] Example 2: N-(2-((4-(4-phenylindoline-1-formyl)benzyl)amino)ethyl)acetamide
[0092]
[0093] ESI-MS m / z:414.2[M+H] + ; 1 H NMR (600MHz, DMSO-d 6 )δ7.81(s,1H),7.54(d,J=7.8Hz,2H),7.46(dd,J=11.5,6.0Hz,6H),7.41–7.35(m,1H),7.29(s,1H ), 7.09(d, J=7.0Hz, 1H), 4.00(t, J=8.0Hz, 2H), 3.76(s, 2H), 3.18–3.09(m, 4H), 2.56(t, J=6.4Hz ,2H),1.79(s,3H),1.23(s,1H).
Embodiment 3
[0094] Example 3: (S)-(4-((2-(Hydroxymethyl)pyrrolidin-1-yl)methyl)phenyl)(4-phenylindoline-1-yl)formyl
[0095]
[0096] ESI-MS m / z:413.2[M+H] + ;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com