Preparation method for phosphate ester
A phosphate ester and phosphorylation technology, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve problems such as deepening chromaticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
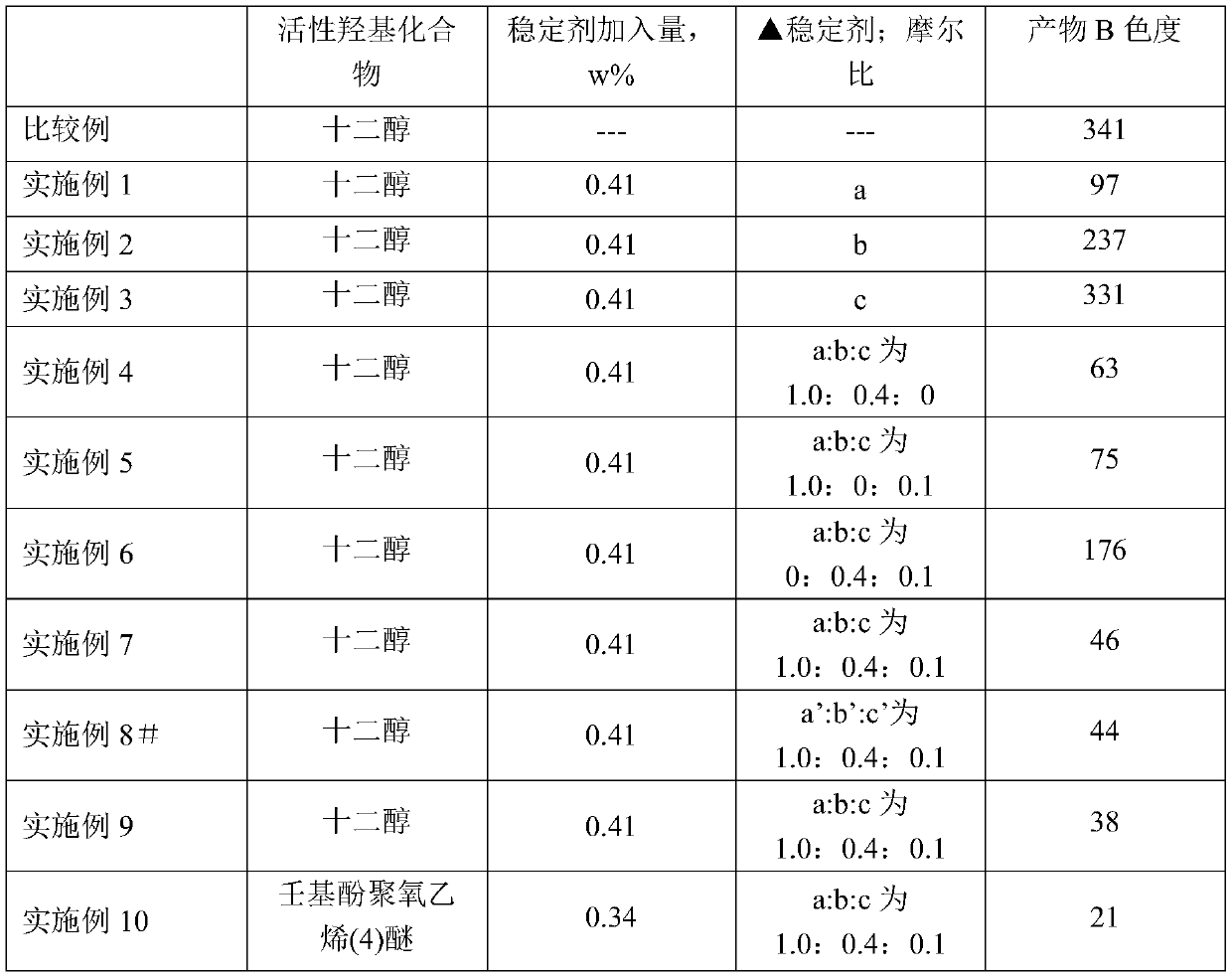
[0044] 1. Phosphate ester preparation
[0045] The difference with the comparative example is that step (2) adds a stabilizer, and the stabilizer is sodium phosphite.
[0046] Add 200.00g (1.08mol) of n-dodecyl alcohol into a 500ml three-neck flask, raise the temperature and turn on the stirring switch; control the temperature at 52±2°C, and add 68.16g of phosphorus pentoxide (0.96mol in terms of phosphoric acid) within half an hour; Nitrogen was filled, and the temperature was kept at 70°C for 2 hours, then 2.68g of pure water and 1.10g of sodium phosphite were added, the temperature was controlled at 70°C, and the temperature was kept at this temperature for 4 hours to obtain the phosphate ester product A. The chromaticity obtained by analyzing the method described in "GB3143-82 Liquid Chemical Product Color Determination Method (Hazen Unit—Platinum-Cobalt Color)" is 38.
[0047] 2. Evaluation of color stability at high temperature
[0048] Add 100 grams of the above-menti...
Embodiment 2
[0050] 1. Phosphate ester preparation
[0051] The difference with the comparative example is that step (2) adds a stabilizer, and the stabilizer is sodium stearate.
[0052] Add 200.00g (1.08mol) of n-dodecyl alcohol into a 500ml three-neck flask, raise the temperature and turn on the stirring switch; control the temperature at 52±2°C, and add 68.16g of phosphorus pentoxide (0.96mol in terms of phosphoric acid) within half an hour; Nitrogen was filled, and the temperature was kept at 70°C for 2 hours, then 2.68g of pure water and 1.10g of sodium stearate were added, the temperature was controlled at 70°C, and the temperature was kept at this temperature for 4 hours to obtain the phosphate ester product A. The chromaticity obtained by analyzing the method described in "GB3143-82 Liquid Chemical Product Color Determination Method (Hazen Unit—Platinum-Cobalt Color)" is 41.
[0053] 2. Evaluation of color stability at high temperature
[0054] Add 100 grams of the above-mention...
Embodiment 3
[0056] 1. Phosphate ester preparation
[0057] The difference with the comparative example is that step (2) adds a stabilizer, and the stabilizer is sodium borate.
[0058] Add 200.00g (1.08mol) of n-dodecyl alcohol into a 500ml three-neck flask, raise the temperature and turn on the stirring switch; control the temperature at 52±2°C, and add 68.16g of phosphorus pentoxide (0.96mol in terms of phosphoric acid) within half an hour; Nitrogen was filled, and the temperature was kept at 70°C for 2 hours, then 2.68g of pure water and 1.10g of sodium borate were added, the temperature was controlled at 70°C, and the temperature was kept at this temperature for 4 hours to obtain the phosphate ester product A. The chromaticity obtained by analyzing the method described in "GB3143-82 Liquid Chemical Product Color Determination Method (Hazen Unit—Platinum-Cobalt Color)" is 41.
[0059] 2. Evaluation of color stability at high temperature
[0060] Add 100 grams of the above-mentioned p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com